Technical Cleanliness Microscope Solutions
In the automotive and electronics industries, particulate contamination in components can lower product performance and lifetime. Similarly, particulate contamination in pharmaceutical products can pose a major risk for patients.
Cleanliness analysis and particle counting solutions from Leica Microsystems help manufacturers achieve technical cleanliness and find the root cause of contamination. These Leica solutions help users keep production efficient and cost-effective.
Contact a local imaging specialist for expert advice on technical cleanliness solutions for your needs and budget.
Can analysis be done with high throughput?
Technical cleanliness analysis can be time-consuming as there are usually many samples or filters to be analyzed. However, it is possible to increase throughput and efficiency by analyzing more in less time.
Leica cleanliness solutions allow you to spend less time on scanning and data analysis with optimized algorithms and combining multiple samples in one batch.
What is the damage potential of a particle?
The harder and larger a particle is, the higher its damage potential. To reliably and accurately assess types of particles, it is essential to differentiate reflective from non-metallic particles and to determine particle size three dimensionally.
Cleanliness analysis and particle counting microscopes from Leica Microsystems help you get more insights on particles and identify the source of particulate contamination.
Why comply with technical cleanliness standards?
Different cleanliness guidelines and standards can be followed to access international or regional markets and also to meet user specifications.
Leica solutions help you to meet these various guidelines and standards, including ISO 16232 and VDA 19 for automotive parts and components, ISO 4406 for lubricants and hydraulic fluids, oils, and USP 788 for pharmaceutical products.
At Allinox, an aerospace company, we have the Cleanliness Analysis Advanced System and use it for analyzing difficult particles on critical parts.
We use this solution to analyze particles as small as 5 µm in size, measure them accurately in 3 dimensions, and distinguish reflective from non-reflective ones in accordance with aerospace standards, e.g., P4TF21.
Since we have this solution, it has helped us to increase the efficiency of our cleanliness analysis and significantly improve the quality and reliability of our products.
Why use Leica microscopes for cleanliness analysis?
Tackle the challenges associated with particle detection. Maintain production speed and cost-effectiveness.
Fast-forward to your results
Perform your particle detection analysis more efficiently by spending less time on scanning and data analysis. Fully automated system settings that are automatically stored and recalled by the software from Leica Microsystems offer you reproducible results.
Efficiently identify particles and analyze your data with fast scanning and processing times, minimum user interaction and a multi-sample holder.
Get more insights on particles
Differentiate between particle types automatically, such as metallic vs. non-metallic and particles vs. fibers. Measure particle height easily with automated tools with the LAS X cleanliness expert software.
Perform contamination control more efficiently with optimized software and a unique 2-in-1 solution using laser spectroscopy (LIBS) for simultaneous visual and chemical analysis.
Work flexibly
Meet guidelines and standards today and in the future with Leica cleanliness solutions. Work with various guidelines and standards such as ISO 16232 and VDA 19 for automotive, ISO 4406 and DIN 51455 for hydraulic fluids and oil, and USP 788 for pharmaceutical manufacturing.
You can also easily remain up to date when international and regional standards change.
Technical Cleanliness: Electronics
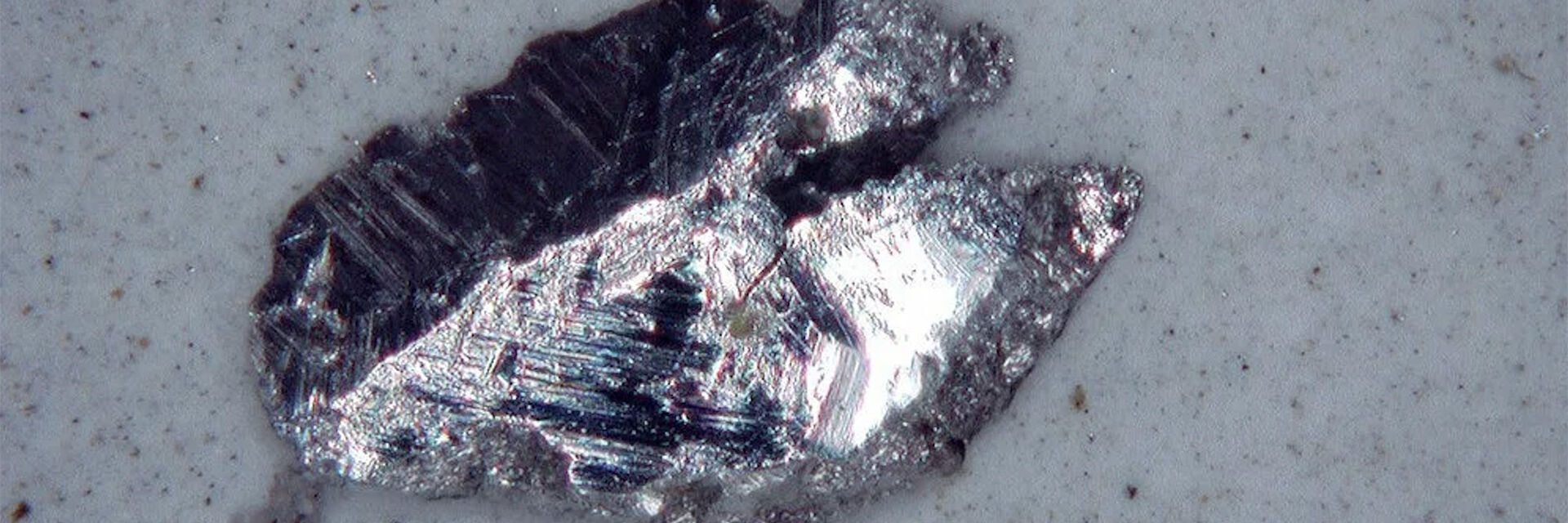
In the electronics industry, component cleanliness is important, because particle contamination can increase the risk of failure, e.g., conducting particles can cause shorts in PCBs. Now with e-mobility, there are also electric vehicles with batteries and electronic parts. A common reference for electronics cleanliness is the ZVEI guideline.
For a cost-effective cleanliness analysis, suppliers and manufacturers must determine efficiently the conductive properties of particles that indicate the potential to cause damage. Automated analysis is key for achieving an efficient process, where normally optical microscopy is applied. Determining the particle composition efficiently with a single solution is a big advantage.
Example: Production of electronic boards
Technical Cleanliness: Automotive and Transportation
In the automotive industry, residual contamination in systems often have an effect on performance and lifetime. A common standards and guideline for automotive cleanliness are ISO 16232 and VDA 19.
To achieve efficient, cost-effective cleanliness analysis, suppliers and product manufacturers must agree on what should be measured to indicate the particle’s potential to cause damage, especially high-risk “killer particles”.
Automated particle analysis is instrumental for an efficient process. Optical microscopy is the broadly applied method. The goal is to find and eliminate the source of contamination. A solution which allows it to be identified efficiently is a big advantage.
Example: Cylinder block from an engine

Technical Cleanliness: Pharmaceuticals
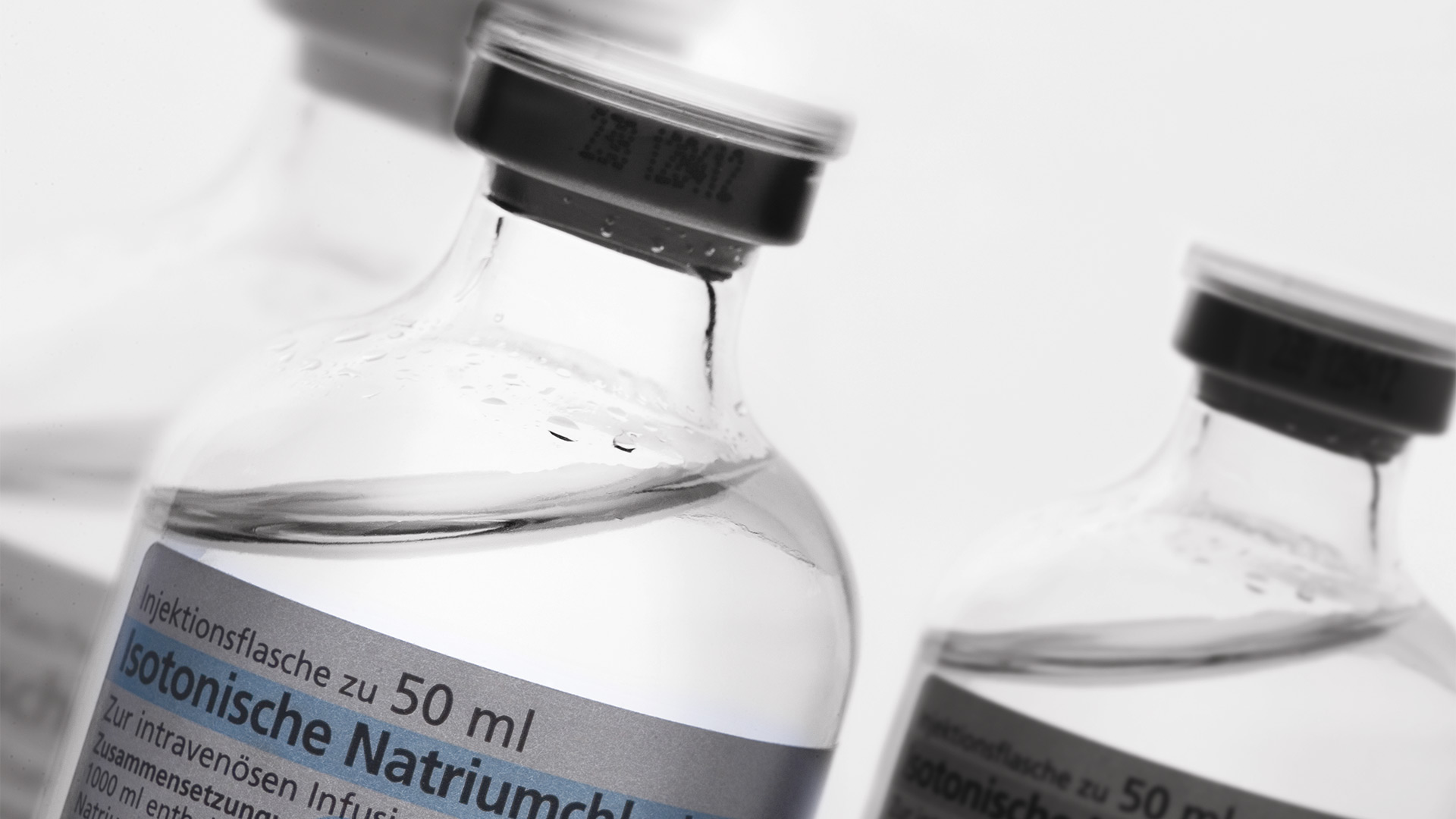
Particulate contamination in pharmaceutical products can come from many different sources. Such contamination can be a risk to patients as they may cause sepsis, inflammatory response, organ dysfunction, phlebitis, and pulmonary arteritis. A standard used in pharma industries is USP 788.
Identification of the particulate contamination is done with optical microscopy. During visual analysis of particles, it can be sometimes difficult to determine the source of the contamination. Elemental/chemical analysis allows the composition to be known, so finding out where they come from (root cause analysis) takes less time and effort. A visual- and chemical-analysis solution offers significant advantages.
Example: Pharmaceutical production of a liquid for intravenous infusion
Comparison table
Emspira 3 | M125 C | DM6 M | DM6 M + LIBS | ||
| Recommended for Particle sizes | Down to 25 µm | Down to 10 µm | Down to 5 µm | Down to 5 µm | |
| Differentiation of metallic and non-metallic particles | Fully automated | Manual | Fully automated | Fully automated | |
| Differentiation between particles and fibers | Fully automated | Fully automated | Fully automated | Fully automated | |
| Measurement of particles | X and Y | X and Y | X, Y and Z | X, Y and Z | |
| Chemical analysis of Particle composition | No | No | No (upgrade possible) | Yes | |
| Recommended for applications | Technical Cleanliness (ISO 16232, VDA 19) | Technical Cleanliness (ISO 16232, VDA 19), Particulate contamination in hydraulic fluids (ISO 4406, DIN 51455), Particulate contamination in pharmaceuticals (USP 788) | |||
Frequently Asked Questions Technical Cleanliness
Yes – report templates are Excel files which can be customized.
Yes, you can measure particle heights with automated tools using the cleanliness expert software. The height is easily determined by using the lowest and highest focus positions for a particle.
No, all Leica technical cleanliness solutions have the ability to autofocus, so proper focus is attained automatically.
By definition the length of a particle is the maximum Feret. For the width of particles the minimum ferret or the maximum inscribed circle diameter can be used.
Yes, it is possible indirectly via the presence or absence of reflection, i.e., discerning between reflective and non-reflective particles. Reflection is ascertained through the use of contrast-enhancing polarization microscopy techniques. Particle composition can also be determined with LIBS (laser-induced breakdown spectroscopy).
Yes, technical cleanliness solutions from Leica Microsystems can be customized to user-defined standards for cleanliness analysis and reporting of results.
Yes, the cleanliness analysis solution can store and recall pre-defined settings for reproducible, reliable results.
The potential of a particle to cause damage can be determined with the use of automated particle differentiation. For example, reflective (often metallic) or non-reflective (often non-metallic) particles, particle heights, and particle composition with LIBS (laser spectroscopy). Normally, harder and larger particles have higher damage potentials.
Yes, with the multi-sample/filter holder from Leica Microsystems you can simplify your workflow by using automated particle analysis.
For cleanliness analysis in the transportation industry, it has become the established practice to follow guidelines, such as VDA 19 from the German Association of the Automotive Industry, for the quantitative determination of particulate contamination on product components.
In the automotive industry, one of the main standards is ISO 16232 which sets out the accepted definitions and ranges of common parameters, e.g., particle class in terms of size, threshold values for particle identification, image settings, etc., used in cleanliness analysis.
The term “technical cleanliness” is used in conjunction with the manufacturing of products and their components in various industries. The product quality can be extremely sensitive to contamination from particles carried in the air or through liquids. As a result, industries like automotive, aerospace, microelectronics, pharmaceuticals, and medical devices have stringent requirements to prevent surface contamination and ensure effective contamination control.
In the electronics industry, a common reference for cleanliness standards is the ZVEI Guideline (ZVEI = German Electrical and Electronic Manufacturers’ Association) entitled “Technical Cleanliness in Electrical Engineering”.
The ISO 4406 international standard specifies the code to define the quantity of solid particles in the hydraulic fluid used in a given hydraulic-fluid power system. The purpose of this code is to simplify the reporting of particle count data by converting the particle number into broad classes or codes, where an increase in one code is generally a doubling of the contamination level.
This standard applies to fresh mineral and synthetic oils with and without additives. Part of the oil sample is filtered through a membrane filter and the residue on the filter is washed oil-free with solvent. The particles on the filter are determined by number and size using an optical microscope. The particle counts are given separately for particle sizes which are coded values according to ISO 4406.
It is a standard concerning particulate matter testing methods applied to injectable fluids and liquids which are used in health care. Examples are intravenous (IV) solutions, injections of medications, etc. Particulate matter refers to undissolved particles that are not intentionally present in injectable fluids which can cause unwanted toxicity or side effects.