Use of fluorescence microscopy for identifying sperm
In order to provide a more scientifically and procedurally robust sperm searching technique, Independent Forensics has developed a fluorescent monoclonal antibody-based kit, SPERM HY-LITER™, for the microscopic identification of sperm from sexual assault evidence. SPERM HY-LITER™ is designed to provide positive identification of sperm using a unique monoclonal antibody that has been chemically tagged with an Alexa 488 fluorophore. The kit incorporates a second fluorescent dye, 4',6-diamidino-2-phenylindole (DAPI) that will stain all cell nuclei; this is a fluorescent analogue of the KPIC stain currently used in most DNA forensic laboratories. By combining both fluorescent dyes, SPERM HY-LITER™ provides several visually confirmatory steps for the identification of sperm. Sperm can be visualized in the fluorescein channel (the fluorescent spectra of Alexa 488 falls conveniently within the emission maximum for fluorescein); all cell nuclei can be seen in the DAPI channel; and using specialized dual filter ‘cubes,’ epithelial nuclei and sperm can be visualized simultaneously.
The monoclonal antibody used in SPERM HY-LITER™ provides an unprecedented degree of specificity that allows the identification of human sperm from previously unsearchable samples. Furthermore, while the signal-to-noise advantage of fluorescent microscopy (and the very low background seen with the fully optimized SPERM HYLITER™ kit) increases the sensitivity of sperm detection by orders of magnitude compared to current brightfield microscopic techniques. The incorporation of both DAPI and Alexa dyes was designed for image processing software such that sperm recognition could be essentially automated. By using computer-aided image analysis software, SPERM HY-LITER™ stained preparations can first be scanned for "features",i.e., fluorescent signals above background – and second, these features can then be analyzed further for the color (or hue) of the observed fluorescence. Only those features that have both DAPI (from the DNA) and the Alexa 488 (from the monoclonal antibody) fluorescence would be scored by the software as sperm.
SPERM HY-LITER™ provides all required solutions for slide staining in pre-calibrated dropper bottles – two slightly different version allow staining of smear slides (often included in sexual assault evidence kits, "rape kits") or of extracts made from evidence swabs or identified stains. The addition of phase contrast to the method, although not required, gives less experienced crime laboratory personnel the ability to visualize cells, nuclei, and sperm in one image.
As an illustration of the specificity and sensitivity of the SPERM HY-LITER™ method, a mixture of sperm from a variety of animal species, with and without human sperm, stained with SPERM HY-LITER™ (Figure 1) is shown.
Sexual assault case study from forensic laboratory
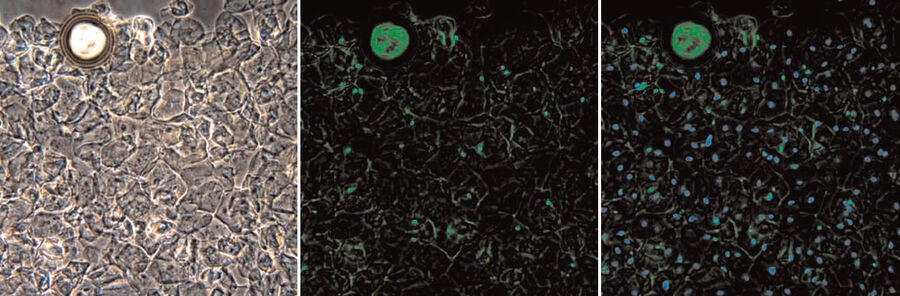
The sensitivity and cell type specificity of SPERM HY-LITER™ is demonstrated from images provided by a crime laboratory case work validation study of SPERM HY-LITER™ (Figure 2). Here a smear slide made by a sexual assault nurse examiner from a vaginal swab collected from a sexual assault victim was stained using SPERM HY-LITER™. These types of slides are notoriously difficult for crime laboratory personnel to analyze for the presence of sperm, as the cell density, collection method, and storage conditions all conspire to destroy sperm cell morphology and inhibit KPIC staining, making standard sperm identification methods all but impossible. The series of images demonstrate the complexity of the original slides (see phase contrast image), the ability to detect sperm in the preparation (see combined phase and FITC image), as well as confirmatory steps in the process where both epithelial and sperm cells can be simultaneously identified (see combined dual cube and phase contrast image). SPERM HY-LITER™ stains sperm in all layers of the preparation.
Efficiently screening evidence
The job of the forensic analyst often involves screening many items of evidence in a case. Although current forensic laboratory protocols vary, screening for sperm is usually performed with 40x objectives (400x final magnification). Here again, SPERM HY-LITER™ provides an advantage over current methods as stained preparation can be easily visualized using 10x and 20x objectives (100x and 200x final magnification) greatly increasing the field of view and therefore decreasing the time needed to scan stained slides. In fact, the signal from SPERM HY-LITER™ stained slides is such that sperm can be scanned using appropriately configured fluorescent-capable stereo microscopes like the M205 FCA or FA (Figure 3). Given the field of view and working distance of these microscopes (and therefore the speed and ease of slide manipulation), this approach could change the way in which crime laboratories search for sperm from sexual assault evidence.