About Roland A. Fleck
ROLAND A. FLECK, PHD, FRCPath, FRMS, is a Professor in Ultrastructural Imaging and Director of the Centre for Ultrastructural Imaging at King's College London. Having specialised in basic research into cellular injury at low temperatures and during cryo-preservation regimes he has developed specialist knowledge of freeze fracture/freeze etch preparation of tissues and wider cryo-microscopic techniques. As director of the Centre for Ultrastructural Imaging he supports advanced three-dimensional studies of cells and tissues by both conventional room temperature and cryo electron microscopy. He is a visiting Professor of the Faculty of Health and Medical Sciences, University of Copenhagen and Professor of the UNESCO Chair in Cryobiology, National Academy of Sciences of Ukraine, Institute for Problems of Cryobiology, Kharkiv, Ukraine.

What is your department doing and what workflows are mainly used in your lab? How does the Leica workflow portfolio support these?
We support all the research activities at Kings College, i.e. principally the life sciences, but we also support materials and chemical science basic research. The workflows we use are a 50/50 split between room temperature and cryo EM. The room temperature research supports diagnostic EM for needle biopsy work that is very straight forward and conventional. There is an EM TP for room temperature sample preparation, followed by an EM UC7 microtome for sectioning and then imaging with the TEM. There is also a lot of more advanced room temperature sample preparation of cells and tissues for sectioning whilst maintaining orientation and then imaging. Samples are also prepared in resin for processes like serial block face scanning microscopy that is again fairly conventional. Although, we do use a Leica vibratome to prepare the tissue.
Moving into high throughput cryo techniques, we do an awful lot of Tokuyasu work, so lightly fixed, but sectioned at -80°C in the ultracut with a cryo box, then immuno-gold-labelled and imaged with the TEM. Then slightly more advanced ones with a high pressure freezing, freeze substitution, immuno-gold-labelling, and tomography workflow. We have additional work which is pure cryo. For example, we have some structural biology, single particle projects, that use the EM GP, blot plunger, and cryo TEM imaging and we also prepare cryo sections of tissues using the CEMOVIS technique. Therefore, high pressure freezing and cryo sectioning, and then cryo TEM workflow. After that it´s the cryo SEM workflow. So essentially, we use high pressure freezing EM ICE, freeze fracture system EM ACE900, EM VCT500 transfer with an EM VCM loading station and a JEOL 7800 prime for the cryo SEM with a VCT cryo stage.
What are the benefits of a cryo SEM investigation?
It is much more rapid. As we showed the other day, I brought a sample into the lab and had a high-resolution image with the SEM in less than two hours. We also had lunch in between. That was quite convenient. So, it is quick and rapid, and the performance of a modern SEM means the information you get from a membrane is as valuable as that from a TEM. You also get topographic information, because of the nature of the signal acquisition in the SEM.
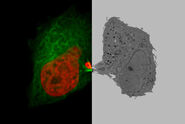
Which types of samples are you typically investigating with the cryo SEM workflow?
Cells and tissues. Cells, in particular, are very interesting. So, any sample which is relatively complex with lots of different organelles and structures which you want to look at with quite high magnifications is ideal to investigate with cryo SEM. For instance, something like a Drosophila embryo would be good, because there is a lot of complexity to the tissue. Malarial parasites inside red blood cells, because it’s an interesting, complicated structure and it’s a little bit too big to get a decent size section into the TEM. The SEM gives you a wider field of view with a high magnification so that’s where your true gain is.
For what research areas are you using the high pressure freezer EM ICE?
The high-pressure freezer is quite useful, because it’s a fairly integral part of the workflow. We have electrical stimulation available on it, which the neuroscientists like. We also have light stimulation which the neuroscientists were using before electrical stimulation came along. We have some interest in re-using light stimulation for other optically responsive samples. We also prepare samples at 6 mm and 3 mm and in interlocking carriers and also with the tube system, so it’s a fairly versatile tool.
Which criteria made you choose the EM ICE?
Service and support. I think it’s actually a high-quality instrument. In my opinion it’s an improvement over the HPM100. In principle, the theory behind it for how it delivers the cooling is the optimal way to deliver cooling and conduct the heat away from the sample. Its more efficient than a turbulent delivery of coolant. I wasn’t so keen on the EM PACT configuration where you had a separation between cooling and pressure. Whereas the EM ICE goes back to the same basic design as the HPM010, but with some improvements. I think it’s good in terms of theoretical and technical design and I get reliable service and support.
How about the usability of the EM ICE?
It’s very easy to train somebody on it. But I think if you are an experienced user on some of the more manual instruments you can be just as quick. The difference to the HPM100 is that I’m faster transferring the sample to LN on an HPM010 consistently, whereas in my view the EM ICE is consistently as quick for the average operator, which is really why it is useful. It’s easy to get everybody working to about the same standard.
Why did you choose to have an EM ACE900 in your lab?
Because having had years of working with freeze fracture systems, I know how versatile they are. It’s a really strong high vacuum cryo processing tool which stands at the middle of most of my workflows. So, I get a really high-quality coating, the chamber is extremely clean, because it is always at high vacuum, and the vacuum-transfer capability into the SEM is outstanding. You don’t have to be a particularly experienced user to get good results from it. That’s really the reason. It’s a very, very powerful piece of kit.
How do you see the future of freeze fracture investigation with cryo SEM, as well as the classic replica TEM workflow?
In my personal opinion there should be more use of cryo SEM with freeze fracture, because the performance of a modern FEG (field emission gun) SEM is so high and so easy to achieve that if you have a robust high pressure freezing, freeze fracture system, cryo transfer system behind it, you should be able to use the system routinely for high resolution work, but you have to understand the workflow. But the workflow now is fairly accessible to the average user, you don’t have to be an expert to make it all work. In terms of classical freeze fracture, my personal feeling is it has a role to play if you are combining it with immunolabelling techniques and more advanced EM techniques, but they are all quite difficult to develop and apply correctly.
Which advantages does the Leica workflow give when used for doing research projects?
The main thing is really, in theory, the compatibility between different instruments when that’s available. The ideal situation is that the planchettes should be matched to the type of freeze fracture work I want to do which should be matched to the stage that’s available to go into the SEM. The same story should be applied if I want to go to freeze substitution for different tissues so it’s really about making sure that every single consumable matches the device and is readily transferrable to the next device in the workflow. And in lots of cases that’s available with Leica Microsystems.