Introduction
Correlative light and electron microscopy (CLEM) is an imaging technique that enables identification and targeting of fluorescently labeled or tagged structures using light microscopy with subsequent imaging at close-to-nanometer resolution using electron microscopy. CLEM is especially convenient for visualization of rare events, which cannot be tracked down by electron microscopy on its own. The combination of two imaging modalities is highly attractive. Where light microscopy falls short of performance, there electron microscopy hurries to help. The resolving power of an electron microscope is about two orders of magnitude greater than that of a conventional optical microscope and, moreover, electron microscopy provides the needed reference space, where both labeled and unlabeled structures can be visually examined. Although the use of CLEM in host-parasite research is not very common today, it fulfills all criteria to become a valuable method for visualization and investigation of the infectious processes, especially in that cases when only a small number of parasitic organisms is needed to cause disease.
We utilized CLEM to investigate interactions between Borrelia burgdorferi and a few mammalian cell lines of neural origin. B. burgdorferi, the spirochetal bacterium, is a zoonotic pathogen whose infectious cycle alternates between an arthropod vector of the Ixodid tick species, and a vertebrate reservoir host. Transmission of B. burgdorferi to humans occurs through the tick bite leading to clinical manifestations associated with Lyme disease. Our goal was to study the tendency of B. burgdorferi to invade non-phagocytic cells, since the problematics around this matter still remains unclear. At the same time, we wanted to find a fast and straightforward procedure omitted of preparation artifacts, which are inherently connected with sample preparation at room temperature, such as those caused for instance by chemical fixation and dehydration [1]. To achieve this, we employed new Leica EM Cryo CLEM system. The fluorescence images captured using this system were correlated with images obtained from a scanning electron microscope operated also at cryogenic temperatures. Imaging under these conditions ensures the best possible sample preservation, very close to a native state.
Results
In order to examine and more fully characterize the events preceding the entry of the pathogen into the host, one has to be able to trace this pathogen on the host surface at first. To achieve this in as short time as possible, light microscopy is a method of choice. We employed Leica EM Cryo CLEM set, a fluorescence microscope with a cryo-stage, a cryo-transfer shuttle cooled with liquid nitrogen allowing an easy transfer of the plunge frozen sample, and a special cryo-objective.
The important prerequisite which allows fluorescent microscopy to be used on a vitrified sample is that the fluorescent label has to be incorporated into the object of interest before this object is vitrified. We used B. burgdorferi carrying a GFP reporter that is stably expressed [2]. The neural cells were seeded onto sapphire discs (Leica Microsystems) and B. burgdorferi spirochetes were added afterwards. Prior to this, for orientation purposes, TEM finder grids (EMS) were placed on the sapphire discs and the discs were carbon-coated. It facilitated the cell-pathogen interaction of interest to be localized with ease in the electron microscope.
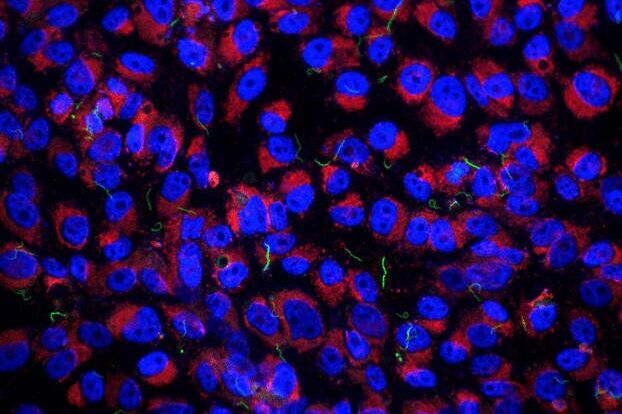
The cells usually grew very dense (Figure 1A) and therefore to find the host cell-bacteria interaction of interest would be quite problematic. To avoid this difficulty, finder grid pattern was created on the sapphire discs (Figure 1B). After finding the interaction of interest and capturing the image with a cryo-fluorescence microscope (Figure 1C), cryo-scanning electron microscopy provided either higher resolution of the point of interest, or reference space (Figure 1D).
Fig. 1: Correlative cryo-fluorescence and cryo-scanning electron microscopy of Borrelia burgdorferi-GFP (green) on the surface of (A) human glioblastoma and (B, C, D) mouse neuroblastoma cells grown on carbon-coated sapphire discs. Cells were counterstained with Hoechst 33342 (blue) and LysoTracker (red).
References
- Dubochet J, and Sartori Blanc N: The cell in absence of aggregation artifacts. Micron 32: 91–99 (2001).
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, and Radolf JD: Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 119 (12): 3652–65 (2009).