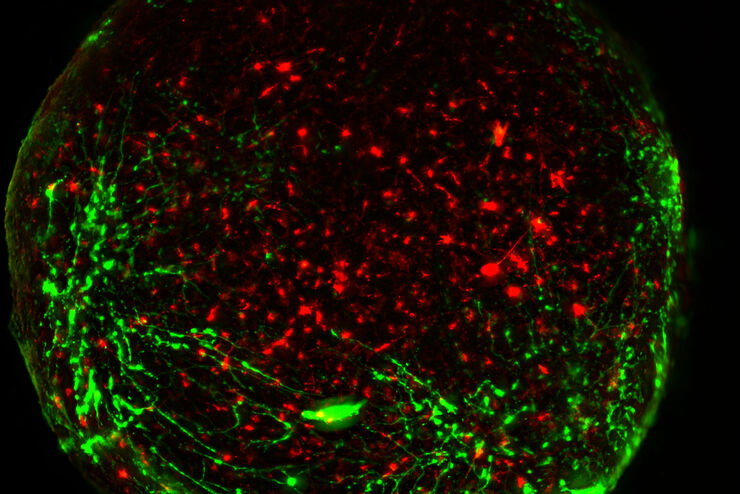
What is Neuroscience?
Neuroscience is a multidisciplinary field involving the study of the structure and function of the nervous system. The purpose is to understand the development of cognitive and behavioral processes as well as understand and find therapies for disorders, such as Alzheimer’s or Parkinson’s disease.
The use of microscopy techniques is critical to visualize the nervous system at cellular and subcellular levels and view any molecular changes within context. Recent developments in deep tissue imaging have provided further insights into neuronal function. Emerging technologies like genetic cell labeling and optogenetics complement these developments.

Techniques for neuroscience research
Research of the nervous system often requires the combination of visualization of large sections of a specimen and high-resolution imaging. Also, it is important to have the flexibility to image different types of specimens, such as live cells, tissues, organoids, and model organisms.
The study of fast dynamic processes, such as cell transport or synaptic remodeling, require high-speed microscopy. One of its main challenges is acquiring high-resolution images while avoiding fluorescence saturation.
To obtain useful data about the nervous system, researchers often use wide-area and volumetric imaging. The need to reduce fluorescence scattering and the background signal can make acquiring images with high contrast and resolution difficult, which is particularly critical when examining neuronal architecture in dense tissues like brain sections.
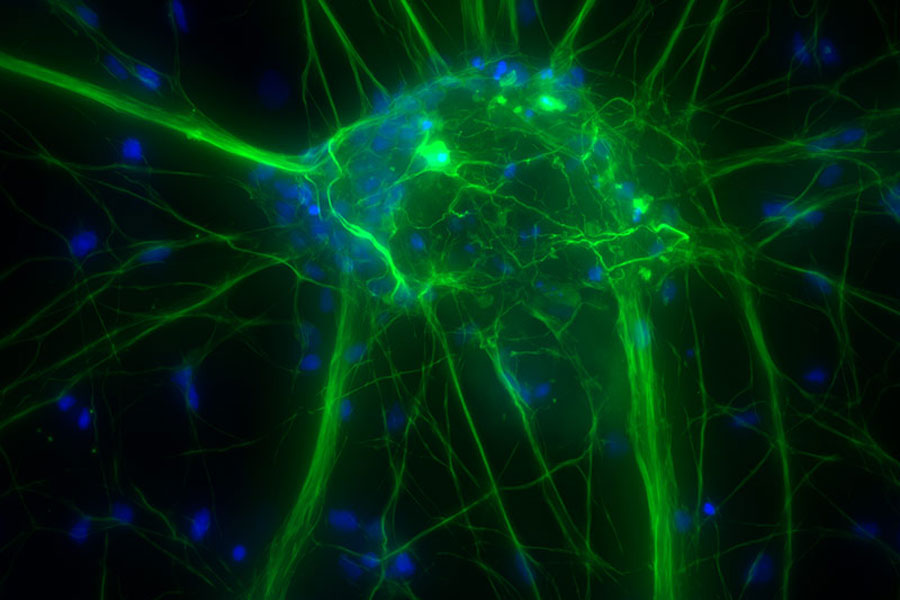
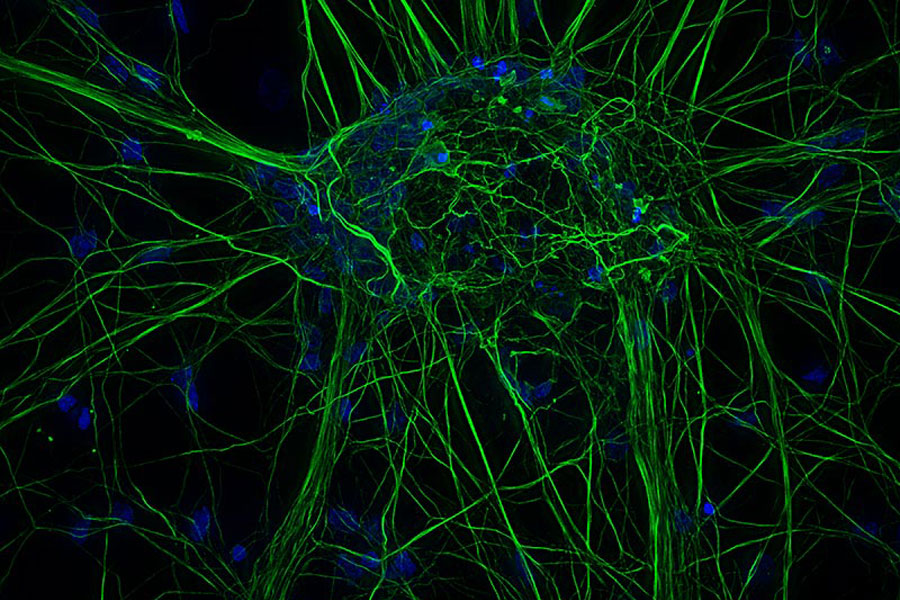
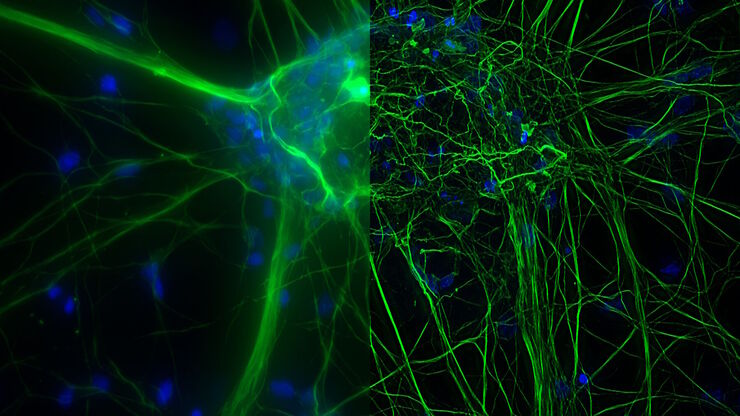
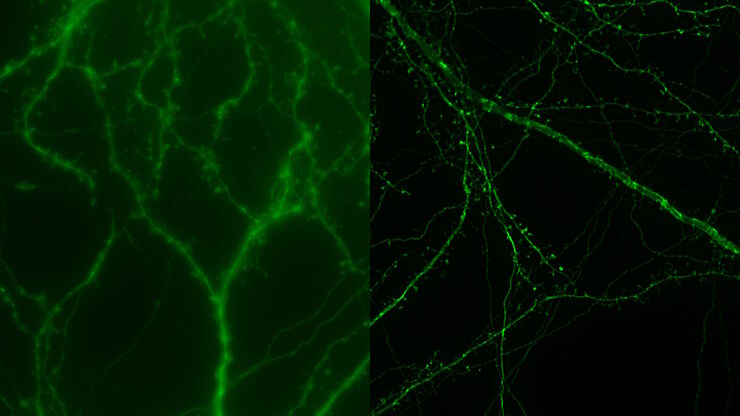
Cultured cortical neurons. Z-stack of 59 planes (thickness: 21µm). Sample courtesy of FAN GmbH, Magdeburg, Germany.
Related articles
What are the Challenges in Neuroscience Microscopy?
Neuroscience Images
How do Cells Talk to Each Other During Neurodevelopment?
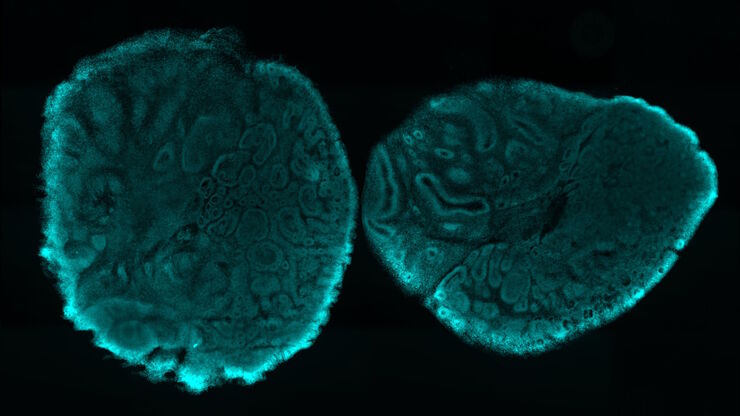
Imaging Organoid Models to Investigate Brain Health
Microscopy methods for neuroscience research
Microscopy methods for neuroscience research
The study of the nervous system typically relies on different types of microscopy for collecting data concerning its activities and structures.
- Multiplexed imaging enables acquisition of information from neurological specimens by visualizing multiple fluorescent biomarkers simultaneously. With this method, biological pathways can be explored at the same time. The advantage is that complex tissue and cell phenotypes can be analyzed and identified.
- Multiphoton microscopy is often used for deep imaging in specimens with minimal invasiveness, thanks to its use of near-infrared excitation of dyes which reduces light scattering.
- Light sheet microscopy is preferred for light-sensitive or thick 3-dimensional specimens. It reduces phototoxicity and photobleaching, while providing intrinsic optical sectioning and 3D imaging.
- Laser microdissection (LMD) is performed with a microscope and laser to prepare neurological specimens for analysis. The heterogeneity of these tissue specimens often requires isolation of specific neurons before analysis can be carried out.
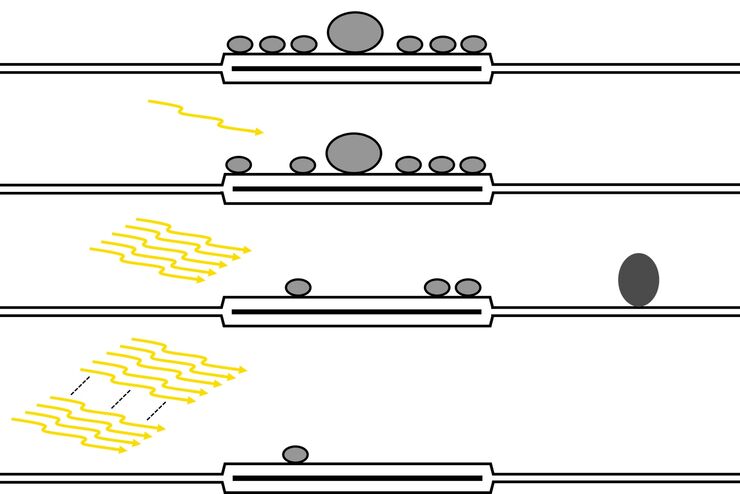
- Electrophysiology is the study of the electrical properties of neurological tissues. The function of nerve cells (neurons) relies on ionic currents flowing through ion channels. Ion channels can be investigated with patch clamping using a microscope and pipette. The electrical activity of neurons can be recorded.
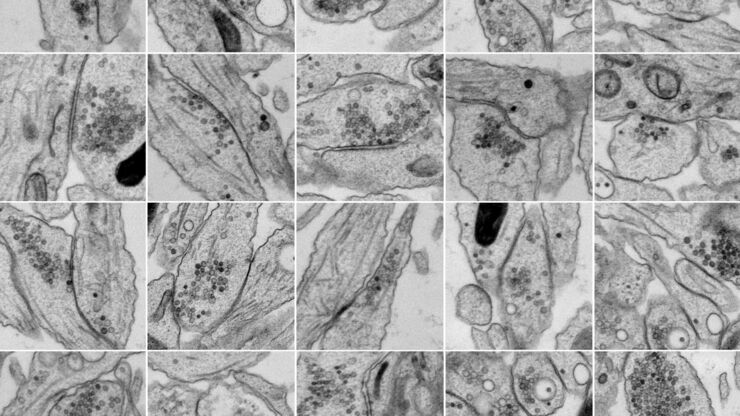
- Electron microscopy (EM) is ideal for imaging neurological specimens with nanometer-scale resolution to better understand structure and function. EM requires special specimen preparation methods, like fixation and sectioning. For neuroscience research enhanced functional EM and optogenetics or electrical stimulation are useful.

Related articles
How did Laser Microdissection enable Pioneering Neuroscience Research?
How Microscopy Helps the Study of Mechanoceptive and Synaptic Pathways
Bridging Structure and Dynamics at the Nanoscale through Optogenetics and Electrical Stimulation
Neuron Isolation in Spatial Context with Laser Microdissection (LMD)
New Standard in Electrophysiology and Deep Tissue Imaging
Investigating Synapses in Brain Slices with Enhanced Functional Electron Microscopy
Neuroscience FAQs
Yes, it is possible with techniques like multiphoton microscopy to image in-vivo brain function.
Innovations like super-resolution microscopy and optogenetics have revolutionized the study of neural networks and cell signaling.
Challenges for optical microscopy include imaging deep brain tissues, reducing light scattering, and achieving high resolution without damaging specimens. For electron microscopy, appropriate preparation of neurological specimens is always the challenge.
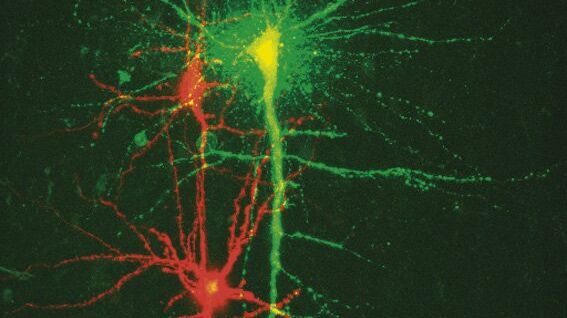
Scientists can observe and analyze the structure, function, and activity of neurons and other cells in the brain with high-resolution fluorescence microscopy. Microscopy plays a key role in helping neuroscientists to better understand how neurons communicate and form networks.
Optical microscope techniques like fluorescence, compound, electrophysiology, confocal, multiphoton, and light-sheet microscopy are widely used for imaging neurons and brain structures. Electron microscopes can also be used after special preparation of the sample.