Introduction
Successful cryo-fixation (vitrification) is the transformation of water from a liquid to an amorphous state without inducing the nucleation of ice crystals. The nucleation of ice crystals is temperature- and pressure-dependent (see diagram below). Crystallisation also depends on the cooling rates as freezing is a time dependant process. The cooling rates depends on the thermal properties of water, the sample thickness and the heat extraction flow at the surface of the specimen.
The idea of freezing biological samples under pressure was first introduced by Moor and Riehle [1]. The High Pressure Freezing device became commercially available in 1985. All High Pressure Freezing machines available currently, despite having different design, deliver synchronized pressurization and cooling of the sample within 20 ms in a highly reproducible manner.
A new dimension in high pressure freezing
Based on the principle of Self Pressurized Rapid Freezing introduced by Leunissen and Yi [3] a new technology has been developed by Leica Microsystems. It uses the tendency for water inside a sealed specimen carrier to expand upon cooling, thereby generating pressure intrinsically instead of using an external hydraulic system. This pressure is likely to be the result of crystalline and low density ice formation within the sealed specimen carrier. To achieve pressure (2,010 bar) where the melting point of ice is lowered to 251 K (Kanno et al. [2]) 60 % of the water inside the specimen carrier needs to be converted to low density ice.
Through the looking glass of physics
Low density ice formation causes a volume expansion relative to liquid water. Numerical simulations show that freezing along the tube walls proceeds freezing in the center, producing a strongly curved ice front. This effect is prominent when the immersion speed is the highest. The separation between regions containing low density ice and well-frozen or vitrified parts is considered to be best when the ice front is as flat as possible. This can be achieved by alteration of the freezing parameters for each specific case within the Leica EM SPF program interface. During the immersion movement, the ice formation front inside the tube is always above the level of the cryogen surface. The distance of this ice front depends on the immersion speed.
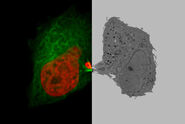
Application – CLEM (Correlative Light Electron Microscopy)
Figure 5: Fluorescent quantum dots inside the cell of interest: The structures containing the fluorescent quantum dots inside the cell of interest were followed live, making a movie sequence with images taken every second. Still images, showing images 5 seconds apart, from this movie sequence are shown. The last image was the last image of the sequence (hence time = 0 s). At this moment the rapid loader was transferred from the stage insert into the RTS and the sample was frozen.
Figure 6: Electron micrograph of the cell of interest (left), zoom into structure of interest (right): Electron micrograph of the cell of interest (left). The boxed area is the area that contains the structure of interest and a zoom into the structure of interest is shown on the right. Arrows indicate quantum dots that can be identified inside the structure. Compare the C-shape of this structure with the last image of Figure 3, and a comparable C-shape can be observed. Note that the electron micrograph is from a 70 nm section while the fluorescent image is approximately 1 μm thick.
Courtesy of Verkade P, University of Bristol, UK.
Related instruments: Leica EM PACT2 with Leica EM RTS
Application – CEMOVIS
The sections are of yeast frozen with a Leica HPM100 in the copper tube system, the cell paste was mixed with a pH 6.5 MES/dextran buffer so that a final MES concentration of 50 mM and a dextran concentration of 20 % was achieved.
The samples were sectioned on a Leica EM UC7/EM FC7 with Micromanipulator at –140 °C and the section thickness was set to 50 nm. The sections were attached to Agar lacey grids.
The sections were imaged using a Tecnai Polara 300KeV (FEI, The Netherlands) microscope fitted with a 4 K Gatan CCD camera. The magnification for the sections was 23 K, with a defocus of –6 um for the tomogram and –8 um for the projection image, and the diffraction was done with a camera length of 930 mm. The image in the left part of the panel is an average of the central 10 slices of a reconstruction done with the IMOD package (Kremer et. al. [4]) image processing software from a tomogram collected using the FEI software.
Courtesy of O'Driscoll J, Clare DK and Saibil H, prepared at the Department of Cryostallography, Birkbeck, University of London.
Applications – Freeze Fracture and Freeze Substitution
Applications – SPF
References
- Moor H, Riehle U.: Snap-freezing under high pressure: A new fixation technique for freeze-etching. Proc. Fourth Europ. Reg. Conf. Elect. Microsc. 2: 33–34 (1968).
- Kanno H, Speedy RJ, Angell CA: Supercooling of water to –92 °C under pressure. Science 189: 880–881 (1975); doi: 10.1126/science.189.4206.880.
- Leunissen JL, Yi H: Self-pressurized rapid freezing (SPRF): a novel cryofixation method for specimen preparation in electron microscopy. J. Microsc. 235: 25–35 (2009).
- Kremer JR, Mastronarde DN and McIntosh JR: Computer visualization of three-dimensional image data using IMOD. Journal of structural biology 116: 71–76 (1996).



![The diagram by Kanno et al. [2] shows the states of water depending on pressure and temperature. At a pressure of 2,045 bar the melting point of water is lowered to 251 K and the temperature for homogenous nucleation is reduced to 181 K. The diagram by Kanno et al. [2] shows the states of water depending on pressure and temperature. At a pressure of 2,045 bar the melting point of water is lowered to 251 K and the temperature for homogenous nucleation is reduced to 181 K.](/fileadmin/_processed_/c/0/csm_Figure-2_14_0fa40e59f6.jpg)