Designing the future of cultivated meat
In order to make cellular agriculture financially viable, raw material costs need to be reduced. Commercial stem cell culture medias can be very expensive, with most containing components unsuitable for food production, often relying on the use of animal origin compounds such as FBS, or small molecules that would be toxic if consumed.
These challenges led Uncommon Bio to take a ground-up approach, using the statistical toolset known as Design of Experiments (DoE) to make cell culture media for cellular agriculture safe and economically viable.
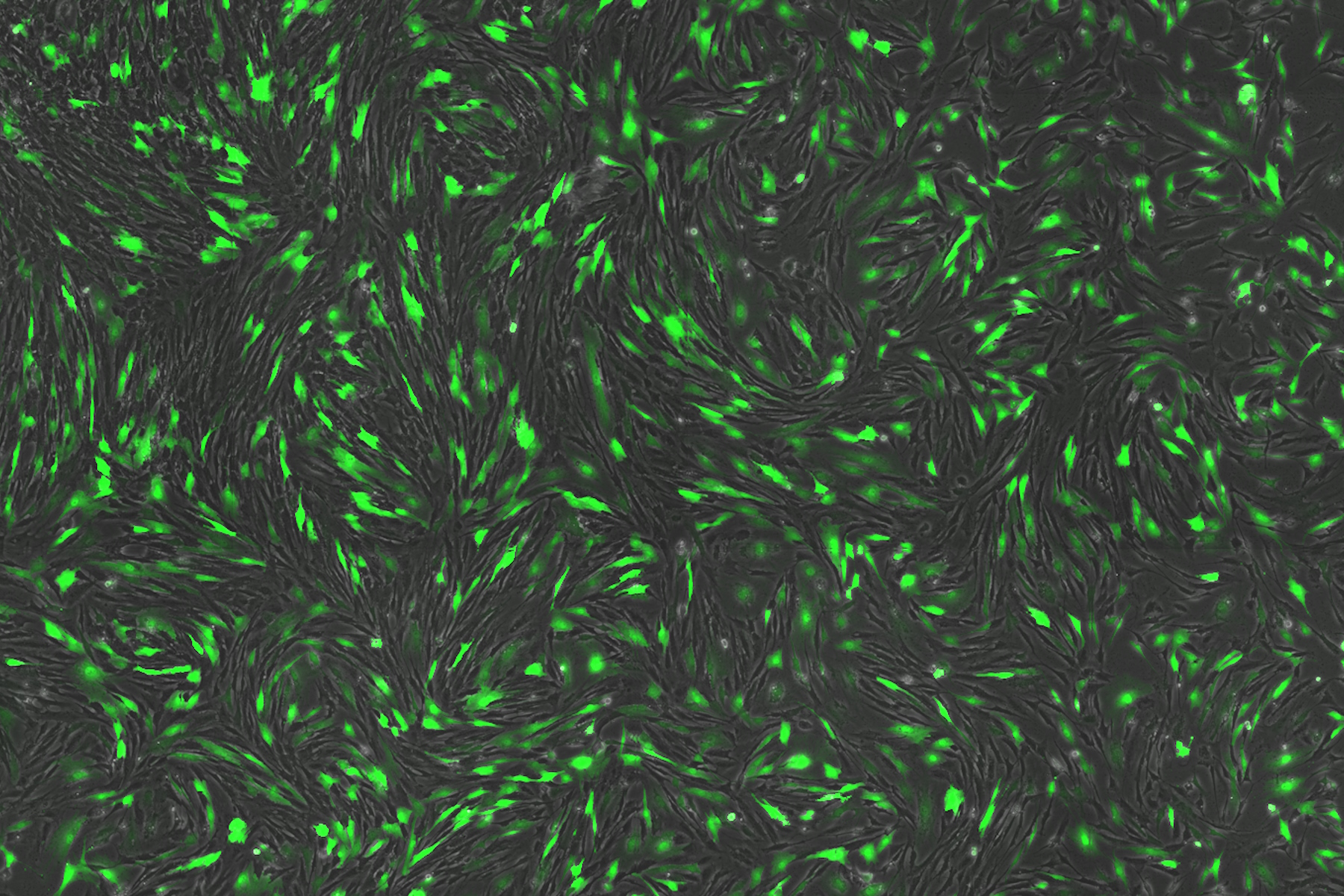
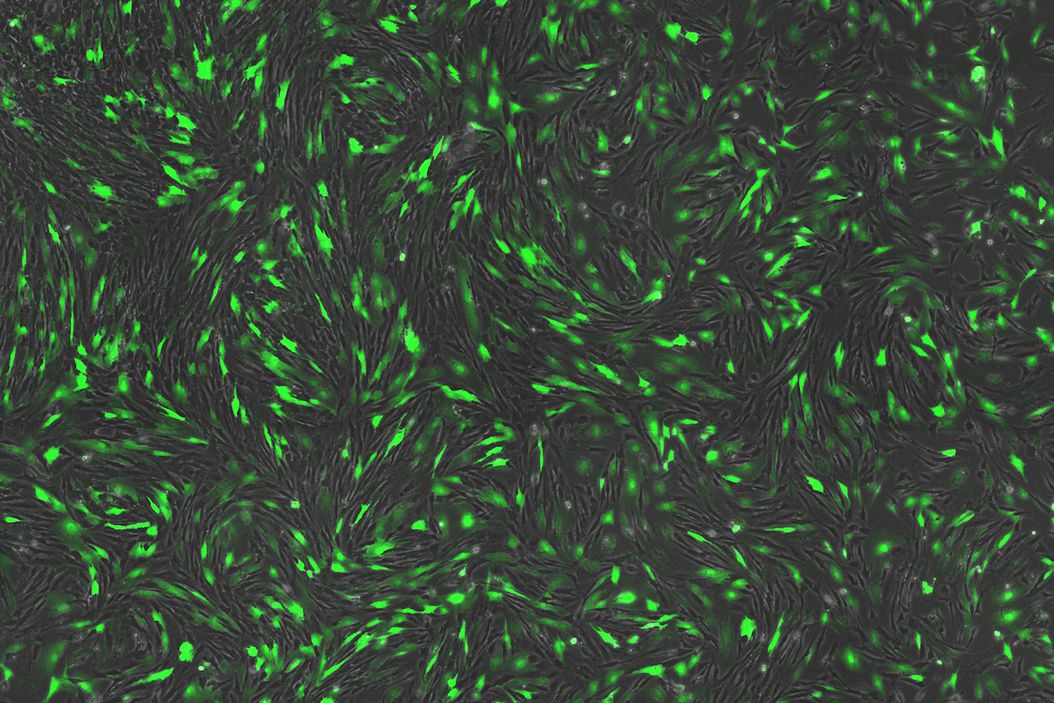
Despite having no previous experience with these technologies, Uncommon have successfully developed a variety of scaled down cell culture models for both adherent and cluster cultures, integrating high-throughput liquid handling and image analysis workflows. These workflows incorporate the THUNDER Imager 3D Cell Culture system, and AI-based machine-learning workflows within Aivia software.
Through these workflows, the team were able to rapidly formulate and quantify thousands of parallel conditions. For media development, this enabled the identification of key interactions and elimination of components that are redundant in the media. As a result, media costs were reduced by 1000x and the team were able to develop animal free, food safe iPSC culture media.
Watch this webinar to see the impact that such high throughput imaging and AI-based analysis, combined with DoE methodologies, can have, and how they can be used to untangle the complex network of interactions that underpin cellular biology.