Challenges when using imaging to study cancer
Optimal temporal and spatial resolution
Research into cancer therapeutics often requires the combination of fluorescence microscopy and innovative functional assays. With optimal temporal and spatial resolution, researchers are able to monitor dynamic events in living cells, such as cell migration and metastasis. These dynamic processes are at the core of cancer development.
Visualizing in real time
Understanding these processes has been challenging due to the difficulty of visualizing tumor cell behavior in real time. Fast imaging over prolonged periods of time tends to come with a sacrifice: either decreased resolution or, more often, harm to your precious specimens. The challenge is finding the imaging technique and system that provides you with the best data with the highest resolution while keeping the cells alive so that you can follow the processes of interest.
Related articles
Empowering Spatial Biology with Open Multiplexing and Cell DIVE
AI-Powered Multiplexed Image Analysis to Explore Colon Adenocarcinoma
A Meta-cancer Analysis of the Tumor Spatial Microenvironment
Spatial Architecture of Tumor and Immune Cells in Tumor Tissues
Uncover the Hidden Complexity of Colon Cancer with Big Data
Multiplexing to understand mechanisms of disease
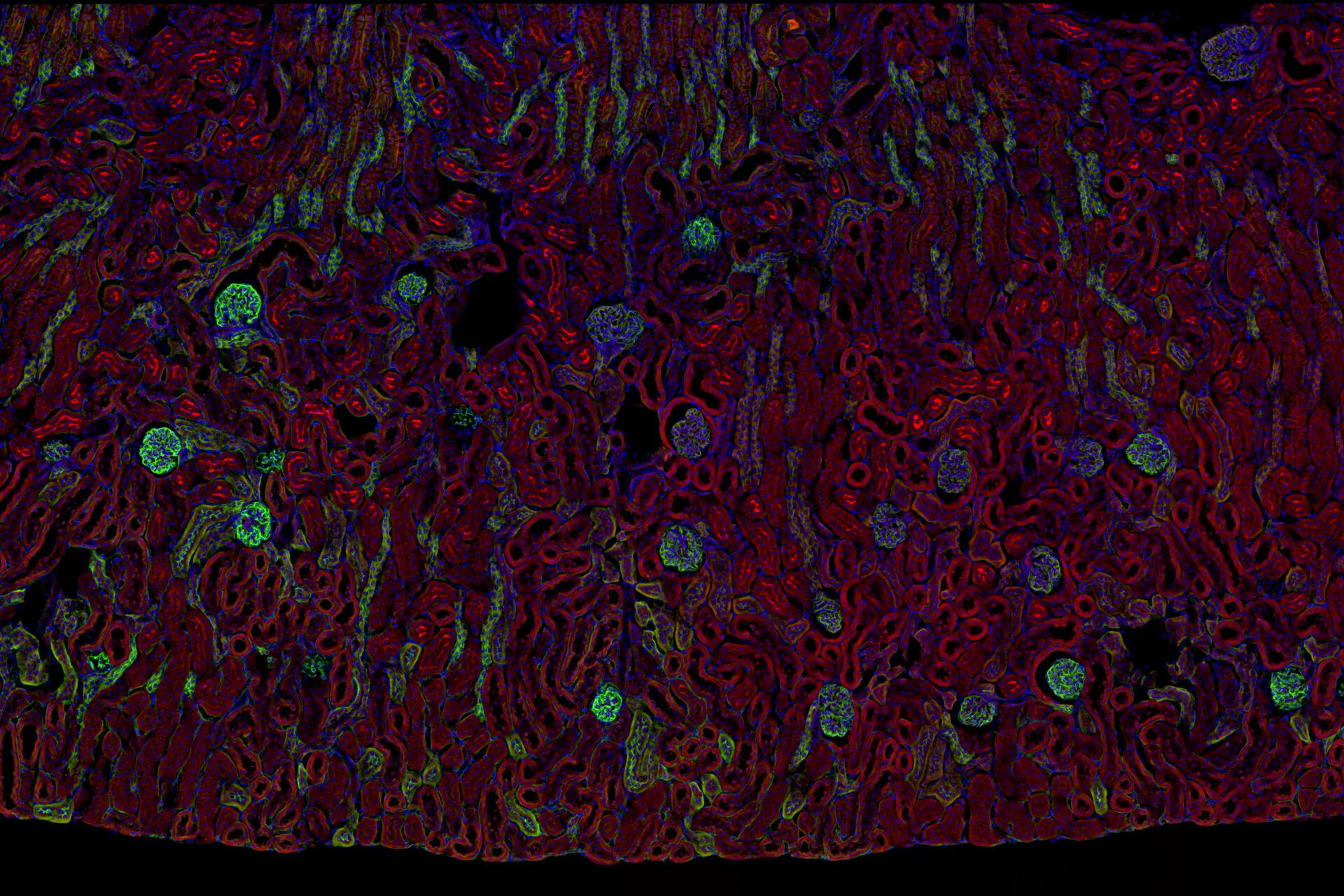
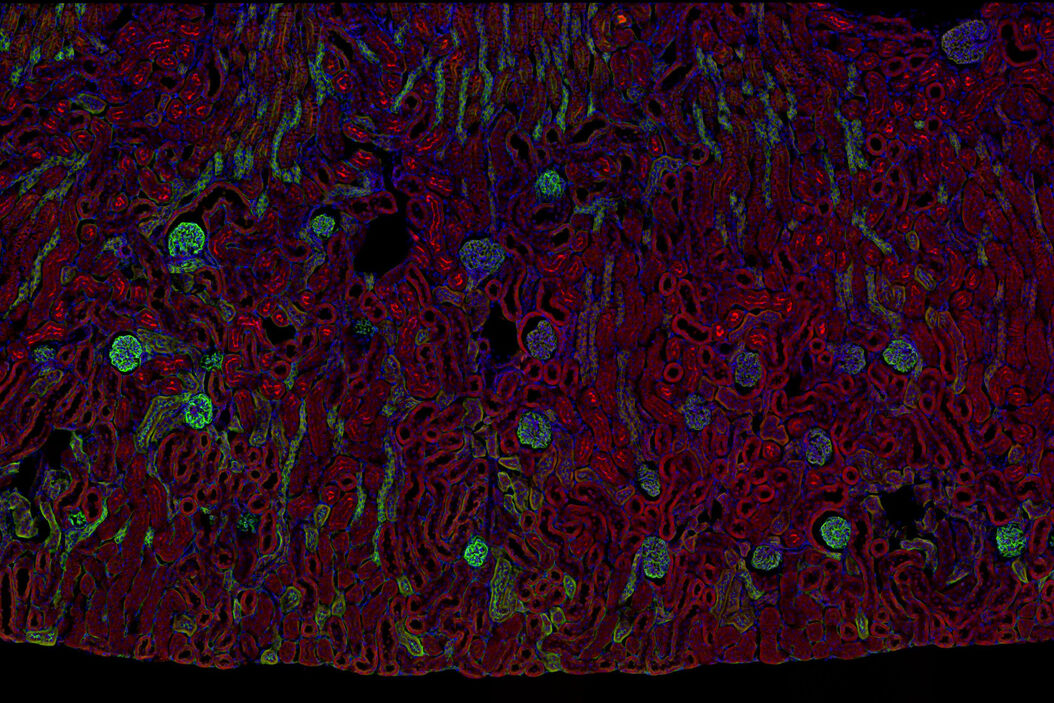
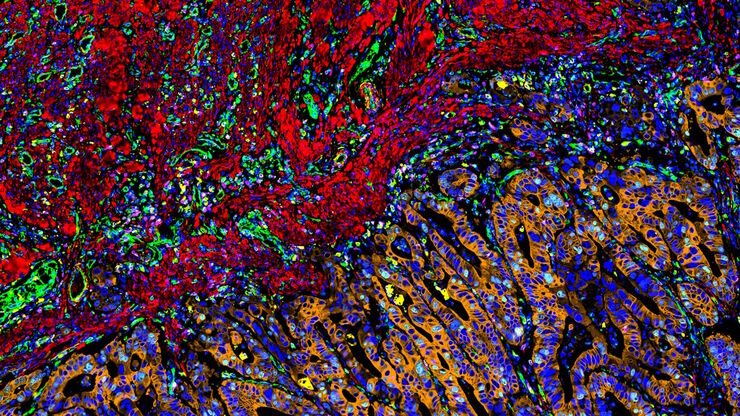
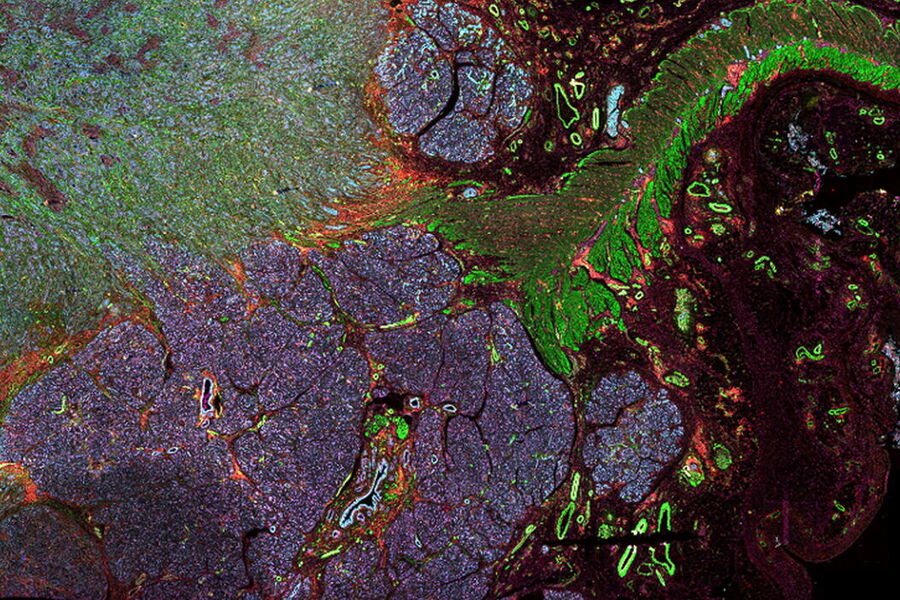
Multicolor fluorescence microscopy, either confocal or widefield based, is a fundamental tool to understand the spatial context, co-localization, and proximity of multiple biomarkers when studying complex events, such as immunosuppression or angiogenesis. This aim can often be challenging, as there are limits to the number of fluorophores you can successfully distinguish with this “multiplexing” approach.
Fortunately, there are innovative imaging systems and strategies to improve the separation of fluorophores (e.g. FluoSync - a streamlined approach for simultaneous multiplex fluorescent imaging using a single exposure) and increase the number of fluorescent probes to that which is needed in your experiment.
Finding the right tools
Cancer is complex and requires a myriad of methods that include spatiotemporally resolved, live-specimen, and single-cell imaging. More insights into cellular processes concerning cancer will come likely from methods with the highest possible resolution and multiparametric image analysis. Approaches like fluorescence confocal microscopy enable you to study multiple targets within tissues or cellular structures.
Advanced imaging techniques, such as super-resolution or, more recently, lifetime imaging or lightsheet, help you to better understand the molecular interactions and regulatory mechanisms behind tumor initiation, progression, and response to treatment.
Laser microdissection or correlative light and electron microscopy (CLEM) enable the study of spatial receptor arrangements in membranes and genome organization in cell nuclei.
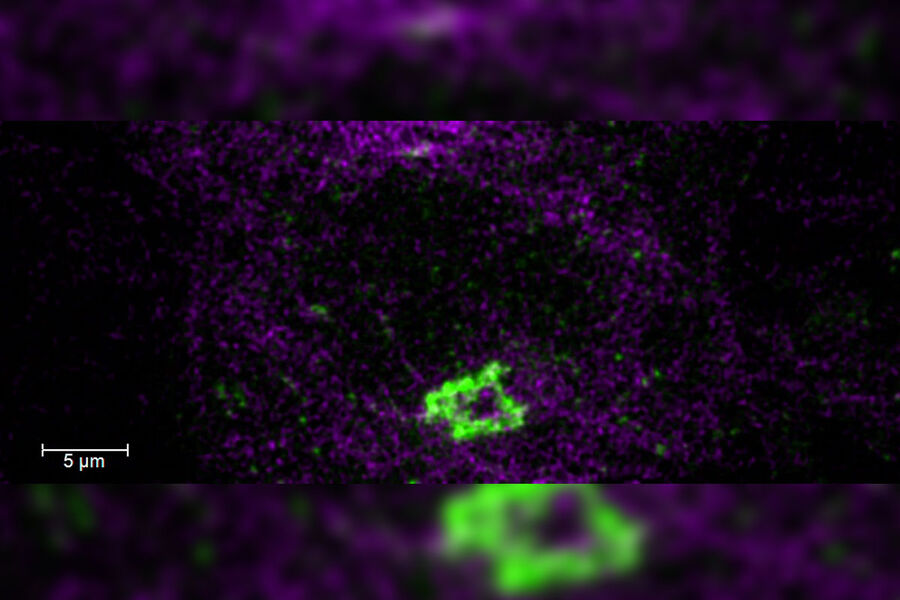
Super-resolution microscopy
Super-resolution light microscopy empowers you to study subcellular structures and dynamics with greater detail. While the spatial resolution of confocal image acquisition is doubled with the LIGHTNING technology, when using STED it can deliver insights at the nanoscale. Learn more here about Leica super-resolution methods and how they enable novel discoveries in the fields of virology, immunology, neuroscience, and cancer research.
Fluorescence microscopy solutions
Find out how fluorescence microscopes from Leica Microsystems support your research. Fluorescence is one of the most commonly used physical phenomena in biological and analytical microscopy for its high sensitivity and high specificity. Fluorescence is a form of luminescence that through microscopy allows users to determine the distribution of a single molecule species, its amount and its localization inside a cell.
Fluorescence lifetime imaging solutions
Leica Microsystems is at the cutting edge of today’s fluorescence lifetime imaging advances. Our systems make lifetime imaging faster and easier to use than ever before, bringing its advantages to every day confocal imaging experiments.