Live-cell imaging
For Coral Life, an optimized light microscopy system that fulfills all the needs for live-cell CLEM experiments has been configured:
- The THUNDER Imager Nano is based on an inverted microscope to enable immersion imaging without the risk of sample contamination by dip-in objectives.
- The THUNDER Imager Nano is an easy-to-use instrument, even for non-experts, providing high performance image data.
- The system uses a highly sensitive sCMOS fluorescence camera allowing you to capture live events with a speed of up to 90 frames per second. Hence, your live-cell CLEM experiments benefit from the following advantages:
- Overview mosaic images of the whole 6 mm diameter sapphire substrate can be acquired quickly at the desired magnification and resolution. For example, a complete sapphire substrate can be covered with 468 exposures using the 40x/1.1 NA objective. The same acquisition with 2 channels (transmitted light and emitted fluorescence) takes around 3 min. Using this approach, manual scanning for suitable regions by eye becomes obsolete.
- Fast cellular events can be captured for subsequent examination by Cryo Electron Microscopy.
- Benefit from the Leica innovative technology Computational Clearing, a part of the THUNDER Imager system. It efficiently removes out-offocus blur, enabling the accurate targeting of proteins or cellular structures of interest for downstream ultrastructural analysis.
Sapphire-optimized imaging
To combine live-cell imaging with cryo-fixation by high-pressure freezing, standard glass substrates are replaced by sapphire discs avoiding breakage of the cell substrate by the induced pressure of 2000 bar. For the Coral Life, we use 6 mm diameter sapphire discs with a thickness of 120 μm. Unfortunately, standard glass-corrected objectives cannot be used, as sapphire glass introduces a chromatic aberration of 80 nm in the visible emission range. To optimize live-cell imaging with sapphire substrates, Leica Microsystems developed a 40x/1.1 NA sapphire-corrected objective for water immersion. This objective lens removes the chromatic aberration caused by the sapphire, while having the same transmission properties as the corresponding glass objective. Furthermore, the objective has a motorized correction collar to compensate for thickness variations of the sapphire.
Haze removal with the THUNDER technology
To identify and resolve target events precisely, best image quality is needed. Unfortunately, in conventional widefield systems background noise, mainly originating from out-of-focus regions can be observed, significantly reducing contrast and the ability to identify structures of interest.
To address this issue, Leica Microsystems developed the THUNDER technology enabling the removal of out-of focus blur. This technology allows the precise targeting of structures of interest for subsequent ultrastructural analysis.
Especially for biological samples, the background is usually not constant throughout the widefield image. It can be quite variable over the field of view. Computational Clearing takes this automatically into account to make the in-focus signals immediately visible.
THUNDER Imagers offer three modes to choose from:
- Instant Computational Clearing (ICC)
- Small Volume Computational Clearing (SVCC)
- Large Volume Computational Clearing (LVCC)
ICC corresponds to computational clearing as described above. SVCC and LVCC are combinations of computational clearing and decision-mask-based 3D deconvolution dedicated to either thin samples (SVCC) or thick samples (LVCC). The adaptive image information extraction of the deconvolution methods follows a concept which evolved from LIGHTNING, Leica Microsystems´ adaptive deconvolution method, originally developed for confocal microscopy.
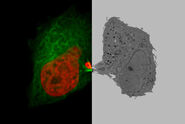
In the following, it is shown that the THUNDER technology works also with sapphire glass as a substrate for different types of samples. All samples were imaged with the 40x/1.1 NA sapphire-corrected water-immersion objective. The first sample is a Hela cell line, highlighting DNA in red and microtubules in green. The task was to follow the different stages in mitosis and cytokinesis to analyze the reconstruction of the mitotic spindle. A proper structural analysis and identification of the target structure is made more difficult due to the inherent background haze. With the application of THUNDER and SVCC, the background noise can be eliminated, and the inherent image information is revealed.
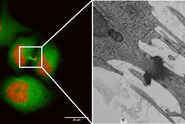
Not only can cells be imaged and processed, but also thicker tissue samples like Drosophila or C. elegans. The next example shows C. elegans embryos labelled with a marker for centrioles (green), i.e., a protein of the pericentriolar material, SPD-5. Here, the centrioles in non-dividing cells of the interphase were targeted. During the interphase, the centriole-GFP signal is dim and, therefore, difficult to identify and discriminate from the background. By using the THUNDER technology, in particular LVCC, the inherent haze could be removed and the best conditions for identification and targeting of these tiny, dim structures were obtained (Fig. 3).
To emphasize the advantage of LVCC for thicker samples, a Drosophila embryo was examined. Centrioles were labelled with Ana-2-GFP to visualize the centrioles dividing in a synchronized manner. The aim was to create a timeline of the centriole division in the EM. For this goal, it was crucial to follow and resolve the centrioles properly. Because of the high fluorescent background of the eggshell, the central signal cannot be so easily detected. The images were processed with ICC and LVCC to remove the background fluorescence of the eggshell and highlight the centrioles.