Why use zebrafish for research?
The zebrafish (Danio rerio), a small freshwater fish, has emerged as a valuable model organism in various fields of life-science research, offering several advantages that complement traditional models like mice and rats:
Ease of Maintenance:
Zebrafish are cost-effective to maintain, requiring minimal space, as they can be housed in small fish tanks that support high-density populations.
Rapid Reproduction & Genetic Modification:
Females can lay hundreds of eggs at once which reach reproductive maturity within 3 to 6 months after fertilization (Hoo et al., 2016). Genetic modifications can be performed quickly and efficiently using a range of established techniques, such as CRISPR and morpholino injections (Ota and Kawahara, 2014).
Transparent Embryos:
Furthermore, zebrafish embryogenesis occurs externally with eggs developing outside of the mother’s body, remaining transparent for an extended period (Kimmel et al., 1995). This transparency allows researchers to directly observe developmental processes, including organ formation, cell migration, and tissue differentiation, without invasive manipulations. Observing embryogenesis without the risk of affecting native development due to physical intervention is a significant advantage over other models where embryo development is hidden from view.
Imaging challenges for zebrafish research
Despite the many advantages of zebrafish, there are some challenges when it comes to imaging, particularly with live specimens:
Size of the Embryo:
Zebrafish embryos can grow to several hundred microns which can be challenging to image even with modern standard microscopes, especially when high resolution is needed.
Movement of Living Embryos:
Live embryos are naturally mobile which complicates image capture. Keeping the embryo still for imaging without restricting its development or behavior remains a challenge.
Overcoming imaging barriers with Mica
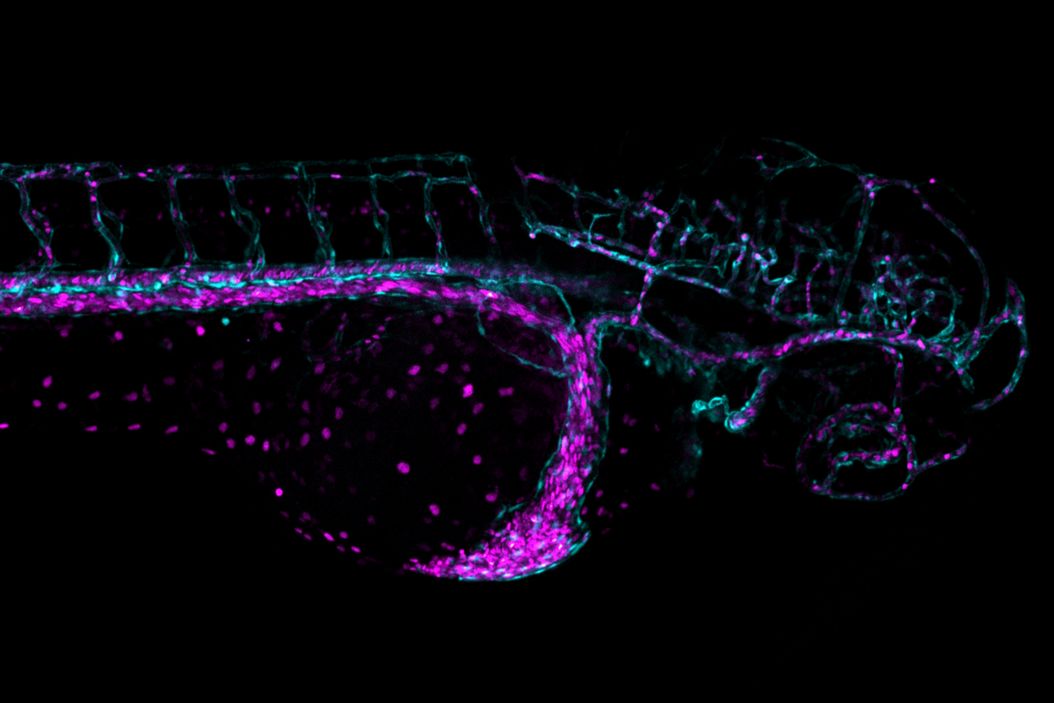
Mica addresses these challenges by enabling high-quality imaging, even under challenging conditions like motion. This case study demonstrates how Mica can overcome some barriers by imaging the blood stream of freely moving zebrafish larvae. The blood stream is visualized with two fluorescent labels: DsRed for erythrocytes and eGFP for blood vessels.
Finding non-immobilized larvae
Traditionally, larvae are immobilized to avoid the tedious process of relocating them. Mica's "Sample Finder" feature simplifies this by generating an overview of the well plate with minimal effort. Navigation for higher magnification imaging is as easy as clicking on the position on the overview or other acquisitions in Mica’s fully integrated Navigator tool. If a larva moves, a spiral scan at low magnification (1.6x) helps quickly locate it, allowing a switch back to higher magnification (10 to 63x) for detailed imaging.
Time-lapse imaging of dynamic events
Mica supports multiple imaging modalities, including transmitted light and high-contrast integrated modulation contrast (IMC; compatible with plastic bottom carriers, Video 1) imaging and fluorescence imaging in both widefield and confocal mode. This versatility allows researchers to choose the most appropriate modality for their experiments.
Long-term high magnification imaging with water immersion objectives (within the limits of the working distance) is further supported by the feedback-driven adaptive immersion ensuring constant immersion.
Video 1: Time-lapse imaging of erythrocyte movement in zebrafish larvae using integrated modulation contrast (IMC). Already IMC alone provides sufficient contrast to visualize dynamic events, such as erythrocytes moving through blood vessels, making it ideal for observing processes label-free.
Widefield imaging is the predominant choice for capturing fast, large-scale events, but the following data (Video 2) clearly shows that, with some constraints, also confocal imaging can achieve comparable high frame rates, but offers improved optical sectioning. Mica's ability to acquire all labels simultaneously eliminates frame rate reduction due to multiple labels and prevents spatiotemporal mismatch.
Video 2: Comparative imaging of zebrafish heart using widefield and confocal microscopy. Time-lapse imaging of a single optical plane through the zebrafish heart using widefield (a) and confocal (b) microscopy. While widefield imaging was performed at 10 frames per second (fps), operating below its maximum capacity, the confocal setup was optimized for speed, imaging with a reduced field of view (FOV) and lower resolution at 23 fps. Cyan indicates blood vessels and magenta erythrocytes.
Volumetric imaging
Capturing 3D information (z-stacks) requires stable larvae to prevent motion-induced data scrambling. Therefore, rapid acquisition is crucial. Mica accelerates the imaging process by capturing all fluorescent channels simultaneously, reducing the likelihood of motion-induced artifacts and speeding up data acquisition.
Video 3: Widefield imaging of zebrafish larvae, moving through the 3D volume, showing blood vessels (cyan) and erythrocytes (magenta).
Mica offers to switch between modalities with a single click, right there on the spot – no transfer of sample to a new system. This ability enables the use of the most suitable modality for the imaging task.
Video 4: Comparison of widefield, THUNDER and confocal imaging modes. While widefield imaging is fast and reveals many details, some structural information remains hidden due to the modality’s inherent out-of-focus blur. Applying THUNDER reduces the out-of-focus information and enhances contrast. Using confocal imaging delivers superior optical sectioning.
Conclusion
Mica is a versatile and powerful imaging system that provides several key benefits for researchers using zebrafish as a model organism, including:
- Efficient localization of freely moving larvae
- Rapid, multicolor imaging without spatiotemporal mismatch between channels
- Seamless switch between widefield and confocal to swiftly adapt to the imaging needs.
Mica greatly enhances the capabilities of zebrafish research, overcoming traditional imaging challenges while enabling detailed observation of complex biological processes.