Studying the Molecular Landscape using Multiplexed Imaging
Alzheimer's disease (AD) is the result of a dynamic interplay among several key molecular factors, including dysfunctional neurons, resident immune cells, microglia, and various immune compartments. The complex and multifaceted interactions among these cellular players may drive the progression of AD, highlighting the importance of understanding the molecular landscape of AD for developing effective therapeutic strategies.
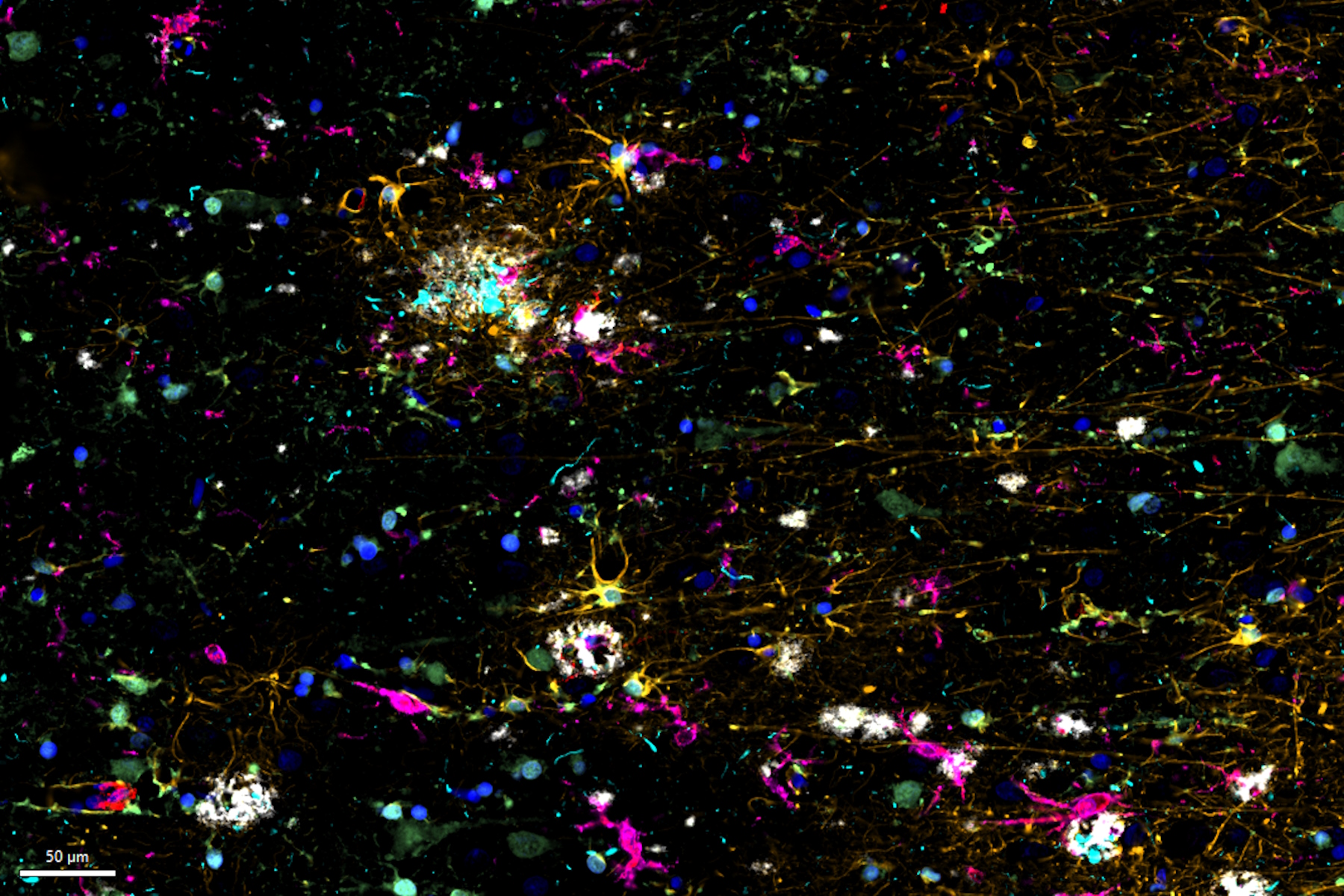
Cell DIVE Multiplexed Imaging Solution combined with IF/IHC-validated antibodies from Cell Signaling Technology (CST), and AI-guided analysis using Aivia enables researchers to investigate the complex neuroimmune interactions, the spatial distribution of immune cells and their influence on AD neuropathology. Cell DIVE is an open multiplexing imaging solution with an iterative staining and dye inactivation workflow that allows imaging of dozens of biomarkers on a single tissue section. Furthermore, this workflow in combination with well-characterized antibodies and AI-guided analysis is crucial for multiplexed cell detection and investigating their co-expression patterns within AD brain tissue.
Overall, multiplexed imaging with Cell DIVE offers a valuable tool to map diverse cellular phenotypes in Alzheimer's tissue, including astrocytes, microglia, AD-associated markers, and immune cell types. By utilizing AI-based analysis tools in Aivia, researchers can identify distinct neuro-immune interactions within AD brain tissue. This enables the investigation of the specific astrocytes, microglia, and immune cell types that surround local β-amyloid plaque neighbourhoods. Through spatial analysis, researchers can understand how immune cell expression profiles differ based on proximity to plaques, highlighting the varied immune dynamics in Alzheimer's disease. These approaches provide valuable insights into the complex cellular and immune interactions in Alzheimer's brain tissue, helping advance our understanding of the disease and potentially identifying new therapeutic targets.