Introduction
Researchers study the mechanisms and cell signaling pathways that control proliferation and migration of smooth muscle cells (SMCs) after vascular injury and during the development of atherosclerosis [1,2]. These cells line the vasculature and are found in every tissue type throughout the body, including but not limited to lungs, intestines, and brain. Published studies report evidence for SMC proliferation and migration in cancer, injury to the eye, and the development of atherosclerosis [1,2]. It is shown here how wound healing of cultured smooth muscle cells in multiwell plates is investigated with less effort and reliably over time using a Leica inverted microscope and on-stage incubator.
Challenges
When a wound is created in a confluent monolayer of cells, the regrowth or “healing” of the wound must be monitored over time to measure the kinetics of the recovery [3]. This requires a reliable imaging solution that can: 1) keep the cells alive for a prolonged period of time and 2) maintain focus on the cells the entire time even when they are imaged in multiwell plates.
Methods
For this study a “wound” was generated in vitro with a confluent monolayer of adherent SMCs in a multiwell plate. The wound was inflicted via a p200 pipette tip. To investigate wound healing, cell growth was then monitored for over 19 hours with a DMi8 S inverted microscope using a 10x/0.32 NA (numerical aperture) objective and phase contrast. The Quantum Stage and a variety of on-stage incubators were used to keep the cell cultures stable [4]. The reproducibility of the stage positioning allows for multiple positions to be captured without losing the location after repeated passes. To maintain focus, the Adaptative Focus Control (AFC) enables the objective to stay “locked” on the sample, as AFC actively checks the sample-objective separation, maintaining focus over time and also from well to well. Images were taken every 20 minutes, resulting in 114 time points. This monitoring was done across 3 wells over a large strip of the total wound area. After acquisition, the images were processed with a Leica 2D-analysis software to calculate the area change over time. A total of 4 wells with different treatments were monitored, enabling multiple wound recoveries to be recorded.
Results
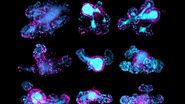
In figure 1, a single position is shown as a representative time series over the 19-hour acquisition and demonstrates typical wound healing progression. The results of area-change-over-time analysis are shown in the bottom row of figure 1. Figure 2 shows data from 3 of the 4 wells plotted as a function of time, indicating the progression of wound healing, i.e., regrowth of the monolayer into the wound.
Conclusions
The results presented here show that the temporal progression of wound healing of cultured smooth muscle cells (SMCs) in a multiwell plate can be studied reliably and easily with a DMi8 S inverted microscope configured with a Quantum Stage, Adaptative Focus Control (AFC), and an on-stage incubator.