Introduction
OCT was first demonstrated in 1991 and was quickly recognized as an important tool in ophthalmology, rapidly becoming the standard of care in retinal diagnostics. Today, OCT is one of the most frequently ordered diagnostic tests. OCT technology is continually evolving, becoming increasingly popular in clinical and non-clinical imaging applications, including ophthalmic surgery, gastrointestinal imaging and interventional cardiology.
OCT functions by a process very similar to ultrasound – except, of course, that is uses light instead of sound. Low intensity, near-infrared (NIR) light is directed at the patient. A small amount of this light is reflected back to the system and detected. The time-of-flight of the returning light is recorded and used to produce an image that shows the subsurface detail of the tissues of interest. In contrast to ultrasound, OCT does not require any physical contact with the patient. It also offers substantially higher resolution, owing to the fact that the wavelength of light is much shorter than the wavelength of sound. Thus, OCT has the capacity to reveal much finer detail than ultrasound.
On the other hand, ultrasound can be used to see deeper into tissue, including structures that are optically opaque. For example, in ophthalmology, OCT is used to visualize fine structures of the cornea or the retina individually, while ocular ultrasound produces coarser images of the entire eye, enabling visualization of large retinal detachments or vitreous hemorrhage.
Advantages of OCT imaging
- OCT systems allows the acquisition and display of high resolution, 3D images in real-time
- State of the art OCT systems can be tailored to a wide range of applications providing sub-micron axial resolution within the eye.
- OCT systems can perform “optical biopsies” of tissue, producing images approaching the resolution of histology without having to resect and histologically process tissue specimens for characterization and diagnosis
- Clinical OCT systems enable non-contact, non-invasive imaging of both the anterior and posterior eye, and come in many forms. These include standard-of-care desktop devices for routine diagnostics, handheld devices for imaging pediatric or supine patients, and microscope-integrated devices for imaging patients undergoing surgery
How can OCT help ophthalmologists acquire high resolution information on ocular tissue?
Optical Coherence Tomography (OCT) is a non-invasive, non-contact imaging modality used to visualize and monitor changes to the morphology of biological tissue. OCT employs low-coherence interferometry to create cross-sectional images that reveal sub-surface details of the tissues of interest.
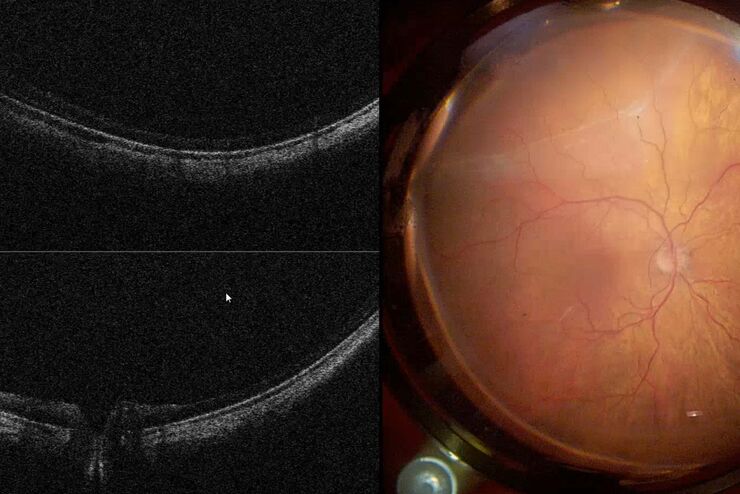
In the most common ophthalmic applications OCT systems use near-infrared light to generate high-resolution, volumetric images of tissue microstructures including the cornea, iris, crystalline lens, vitreous and retina. These images can enhance insight into pathological conditions such as glaucoma, age-related macular degeneration or diabetic retinopathy.
Related Articles
What is intraoperative OCT telling us?
How Intraoperative OCT Helps Gain Greater Insight in Glaucoma Surgery
Posterior Segment Surgery: Benefits of Utilizing Intraoperative OCT
Intraoperative OCT in Retinal Procedures
“Coherence” in Optical Coherence Tomography
Coherence refers to the ability of light to interfere. In an interferometer, light from a single light source is split by a beamsplitter into two paths. After traversing these two paths, the light is then recombined and directed towards a detector. If the light emerging from each path is coherent, an interference signal will be observed at this detector.
In an OCT system, such an interferometer is used and is illuminated by a “low-coherence” light source. These light sources will only produce interference if the path traveled by the light returning from the sample and reference paths matches to within a coherence length, which is typically a few microns. The fact that this light only interferes under these very precise conditions is what gives OCT its very fine axial resolution. The coherence length of the light source is related to its optical bandwidth; that is, how many wavelengths (or colors) the light source emits. The broader the bandwidth, the lower the coherence, and thus the better the axial resolution.
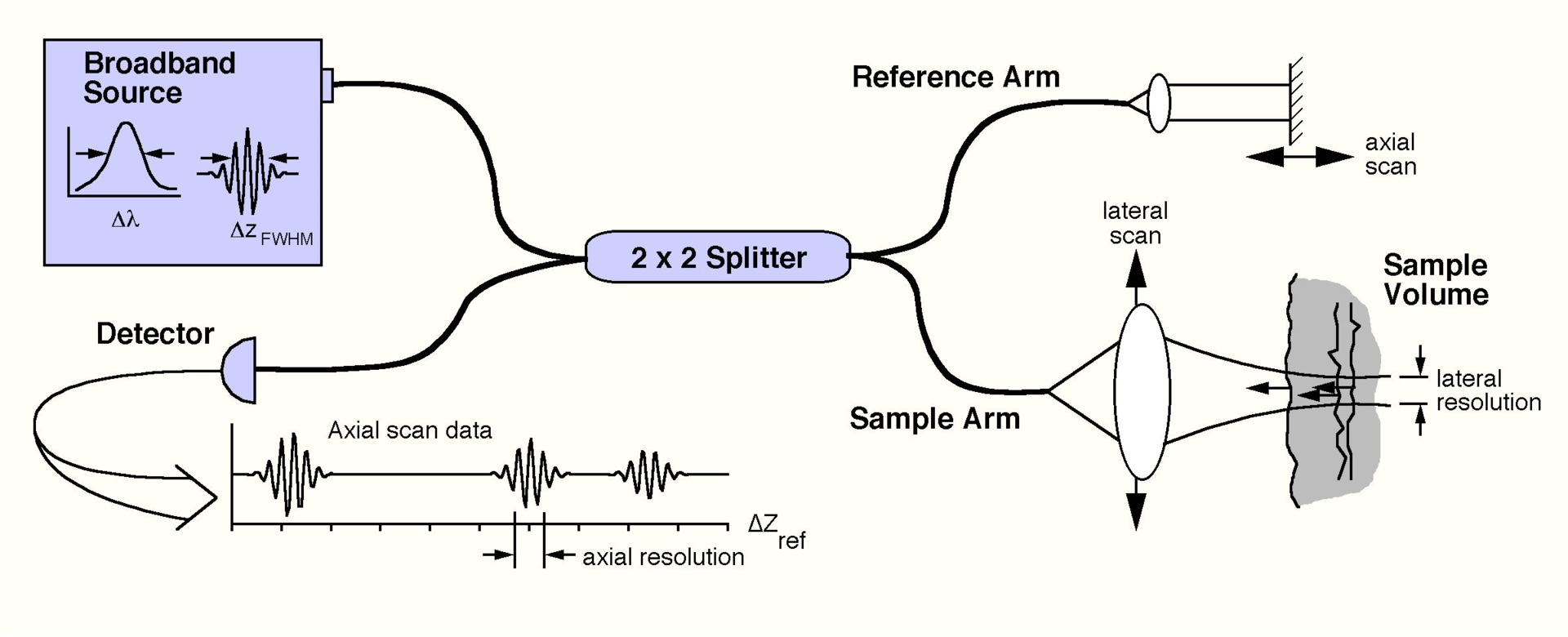
A schematic of an OCT system is shown below. Here, light from a broadband source is split into two paths by a fiber coupler (a convenient, fiber-based beamsplitter). One of the beams is direct towards the sample to be imaged, while the second beam is directed towards a reference arm with a variable path length. The backscattered light from the sample is mixed with reflected light from the reference arm and detected at the output of the interferometer. Because the light source is a “low-coherence” source, interference fringes will only be observed if the length of the sample and reference paths match to within the coherence length of the light. The time delay and intensity of backscattered light from sites within the sample can be measured by detecting the interference output of the interferometer, while varying the reference path length. This embodiment of OCT is known as time-domain OCT, because the reference arm length is varied as a function of time.
“Tomography” in Optical Coherence Tomography
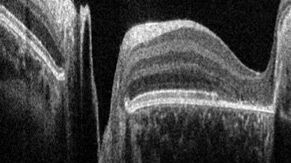
Optical Coherence Tomography is so called because it produces tomograms; that is, cross-sectional slices that can be combined to build up a volumetric picture of an object. In OCT, these tomograms are produced by scanning light across the subject to be imaged. At each location, a depth profile, or A-scan, is produced from the time delay and intensity of the backscattered light. Each A-scan provides information about the reflective or scattering properties of the subject as a function of depth at one position of the scanned beam.
A cross-sectional image is then produced by scanning the beam across the sample and assembling a collection of neighboring A-scans. This produces the tomogram, or cross-sectional slice of the tissue, commonly known as a B-scan. Typically, we think of a B-scan as being an image of a planar slice into the subject, similar to what you would observe in a single histological slice through the tissue (but obtained non-invasively).
A volumetric image is then constructed from a collection of B-scans. There are three major types of volumetric images used in ophthalmic OCT imaging:
- Rectangular, or raster volume scan: A series of parallel B-scans
- Radial volume scan: A series of B-scans at regular angular intervals
- Annular volume scan: A series of B-scans forming concentric rings
Each of these scan types is useful in particular circumstances. For example, rectangular volumes are most frequently used in imaging the macula, annular scans are often used to image in the vicinity of the optic nerve head, and radial scans are most useful for imaging the cornea and anterior segment of the eye.
Spectral Domain OCT (SDOCT)
Spectral Domain Optical Coherence Tomography (SDOCT) is a much more efficient form of OCT. It differs from time domain OCT in that an SDOCT system detects all of the wavelengths of the light in the detection arm of the interferometer individually, using a specially designed, high-speed spectrometer. In these systems, the reference arm remains stationary, and the depth profile (A-scan) of the tissue is obtained by taking the Fourier transform of the spectrum of the returning light, known as the spectral interferogram. This conveniently allows the OCT system to detect light from all depths within a sample at once, rather than just the depth that is matched to the reference arm. As a result, SDOCT systems are much faster and produce much brighter images than their time-domain counterparts.
Through judicious design of the spectrometer, selection of the light source, and implementation of mathematical techniques, SDOCT systems can be tailored to provide exceptional images for a wide range of research and clinical applications. EnFocus intrasurgical OCT and Envisu clinical and pre-clinical OCT systems from Leica Microsystems employ SDOCT.
Related Articles
Ophthalmic Gene Therapy Subretinal Injection
Moving to Routine Use of Intraoperative OCT in Eye Surgery
Towards Advanced Use of Intraoperative OCT in Cataract Surgery
Applications for OCT
High resolution, rapid, and non-contact OCT imaging is used in pre-clinical ophthalmic research, clinical diagnosis and now in ophthalmic surgery. Preclinical OCT imaging systems, such as the Envisu R-Class from Leica, support small to large animal eye research, providing vital information on important pathologies and during the drug discovery process. Clinical OCT systems, such as the Envisu C-Class, aid the diagnosis of physiological and pathological eye conditions. Most recently intrasurgical OCT, for example the EnFocus from Leica, has been introduced to provide visual anatomic feedback to surgeons during ophthalmic procedures, helping to guide surgical decision-making in anterior and posterior segment cases.