Introduction
The interest in gene therapy applications for retinal dystrophies has exploded from the FDA approval of voretigene neparvovec-rzyl (Luxturna®) for the treatment of retinitis pigmentosa and type 2 Leber Congenital Amaurosis.10-14 Growth in this budding field has been prolific. There are currently over 30 gene therapy trials either completed or actively recruiting, and the list of indications is ever expanding. Most of these trials utilize a technique of subretinal injection of adeno-associated viral (AAV) vectors for transfection of photoreceptors or the retinal pigment epithelium (RPE). Intravitreal administration of AAV vectors is limited by dilution into the vitreous cavity, the natural barrier function of internal limiting membrane, and high off-target transfection with risk for an inflammatory response to the vector which is dose-dependent.
The surgical technique for subretinal injection of gene therapy is technically demanding and challenging due to the difficulty of identifying cannula depth and penetration while monitoring fluid distribution of bleb formation and propagation [15-17]. The surgeon’s hands must be steady and make only small adjustments for approximately two to five minutes while varying injection pressure and adjusting microscope controls with foot pedals. Potential complications include inadequate or unsuccessful treatment, escape of vector from the bleb, or surgical trauma. Inadequate treatment may occur from failure to deliver the full amount of vector, failure to cover the desired treatment area, or delivery of vector into the wrong ocular layer (i.e. suprachoroidal instead of subretinal). Vector reflux occurs with enlargement of the retinotomy, inadvertent creation of a second retinotomy when attempting to engage the primary retinotomy, or merging of blebs when multiple blebs are created. Surgical trauma results when high injection pressures place excessive stress on the RPE or fovea causing permanent RPE deformations or full thickness macular hole. Intraoperative optical coherence tomography (Intraoperative OCT) provides real-time information and surgeon feedback about depth of penetration into posterior segment structures, the distribution and propagation of injected fluid, vector reflux, and the integrity of the fovea during these maneuvers. This improves the safety of the procedure and accuracy of treatment delivery in retinal gene augmentation therapy.
Methods
A retrospective consecutive chart review was performed on all eyes of patients who underwent gene augmentation therapy surgeries with or without intraoperative OCT at the Cincinnati Eye Institute (CEI) or Cincinnati Children’s Hospital Medical Center (CCHMC) between September 26, 2018 and March 26, 2020. The study was performed in accordance with the guidelines of the Declaration of Helsinki. Voretigene neparvovec-rzyl (Luxturna®) was exclusively performed at CCHMC for retinitis pigmentosa (RP) or Leber congenital amaurosis (LCA) type 2 associated with biallelic RPE65 mutations. All other procedures were performed at CEI in the context of open-label prospective interventional clinical trials for various indications including achromatopsia (biallelic CNGA3 or CNGB3), and X-linked retinitis pigmentosa (RPGR): NCT02935517, NCT02599922, and NCT03316560, respectively. Electronic medical records were reviewed for demographics, indication, visual acuity, manifest refraction, intraocular pressure, retinal imaging, OCT and fundus autofluorescence on Heidelberg Spectralis® HRA-OCT (Heidelberg Engineering, Heidelberg, Germany) and ultra-widefield fundus photography and fundus autofluorescence on Optos California® (Optos Inc, Malborough, MA). Surgical videos and operative reports were reviewed for injection volumes and pressures, bleb locations, adequacy of treatment area coverage, and for surgical complications.
Surgical Procedure
Gene augmentation therapy was delivered under general anesthesia via subretinal injection after 23-gauge pars plana vitrectomy (PPV) performed on the Alcon Constellation® System (Alcon Laboratories, Ft Worth, TX). The posterior hyaloid face was attached before surgery and manually elevated with the vitreous cutter, nitinol loop (Alcon Surgical Finesse® Flex Loop), or membrane pick. Successful vitreous dissection was confirmed with intravitreal preservative-free triamcinolone acetonide (Triesence®). Epiretinal membrane removal and ILM staining was not required. In some cases, the nitinol loop was used to perform partial ILM peeling at the retinotomy site to ensure complete removal of membranes that might prevent initial bleb formation. The subretinal injection was performed via a 41g subretinal cannula (DORC 1270.EXT, MedOne PolyTip® 23/38g or 25/28g, or Synergetics/Bausch & Lomb 25/41g) on a viscous fluid controlled (VFC) MicroDoseTM Injection Kit (MedOne Surgical, Sarasota, FL). A balanced salt solution (BSS) pre-bleb was required on most study-related procedures prior to vector injection. The subretinal cannula tip was beveled for all Luxturna® injections and all pre-blebs to facilitate entry into the subretinal space. Vector injection after the pre-bleb was performed with an unbeveled subretinal cannula. Fluid-air exchange was performed on eyes with RP or LCA type 2, to tamponade retinal breaks, or to migrate the treatment bleb posteriorly to cover the desired treatment area. All patients regardless of indication received perioperative oral corticosteroids, retrobulbar or posterior subTenon’s triamcinolone acetonide (Kenalog®) depot injection (40mg in 1 mL), and topical corticosteroids and antibiotics. Some patients received oral acetazolamide or topical dorzolamide to prevent or reduce persistent subretinal fluid after subretinal injection.
Intraoperative OCT
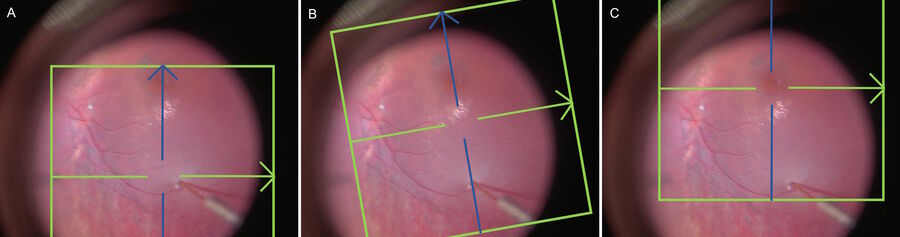
Surgery was performed with the Leica M844 or Proveo 8 microscope (Leica Microsystems, Wetzlar, Germany) integrated with the BIOM® 5 (Oculus Surgical, Port St. Lucie, FL). Intraoperative OCT was performed when available with the Leica Proveo 8 with EnFocus UltraHD spectral domain high resolution OCT. Auto-locate, auto-sharpen, and auto-brighten functions were used to optimize OCT image quality. Image injection provided real-time feedback during the procedures and the intraoperative OCT can be controlled via the foot pedal by the primary surgeon. Volume scans under the widefield viewing system and flat contact lens were used to document pre- and post-injection anatomy. Crosshair rasters were generally utilized to simultaneously monitor the retinotomy and fovea during bleb creation, although a single raster scan could monitor both locations in some cases (Figure 1).
Statistical Analysis
ETDRS visual acuities were converted to Snellen and analyzed as logMAR visual acuities. Statistical analysis was performed with the student t-test using Excel v16.39 (Microsoft© 2020). The null hypothesis was rejected if p<0.05.
visualizing the retina in real time)
Results
Baseline demographics are presented in Table 1. Thirty-four eyes of 26 patients underwent gene augmentation therapy by vitrectomy with subretinal injection during the study period. All eyes had successful subretinal injection of the appropriate vector. The primary retinotomy was created along the superotemporal retinotomy in all cases. A second retinotomy along the inferotemporal arcade and more posterior was required in two eyes when the initial bleb propagated away from the fovea, which was the intended treatment area. Two eyes required fluid-air exchange to migrate vector posteriorly into the fovea/treatment area.
Eighteen eyes of 16 patients were performed with the aid of intraoperative optical coherence tomography, and 16 eyes of 10 patients were performed without intraoperative OCT. Blebs in three eyes treated without intraoperative OCT failed to reach the fovea after initial subretinal injection. Although foveal treatment was not mandated and initially discouraged for RPE65 eyes, visual acuity has improved in younger patients where the fovea was treated with Luxturna®. Only one eye in the intraoperative OCT cohort required an additional maneuver beyond formation of a single bleb, and that was related to failure of the pre-bleb to propagate in an eye with achromatopsia.
Macular surgical complications were observed with greater severity and frequency when intraoperative OCT was not used during subretinal gene augmentation surgery (37.5% vs 5.6%, p=0.021). For surgeries without intraoperative OCT, these included RPE stretching or tearing (n=3) and lost ellipsoid zone (n=3). One eye experienced limited RPE stretching with minimal residual tension line. Retinotomy locations initiated closer to the fovea reached the fovea at lower volumes injected but also subjected the fovea to higher injection pressures. This was due both to the initial proximity of the retinotomy when considering the distance as a radius for a spherical cap formula and local structural differences in photoreceptor integrity in diseases with centrifugal patterns of retinal degeneration (i.e. rod-mediated diseases).
Unfortunately, at the present time the variety of treatment indications and limited and disparate follow-up (median 53 and 250 days with and without intraoperative OCT, respectively) precludes long-term efficacy outcome analysis by comparison of visual acuity or OCT characteristics.
Discussion
Successful gene augmentation therapy by transvitreal subretinal injection depends upon accurate delivery of the intended quantity of treatment vector to the desired treatment area with limited trauma to ocular structures. Although not strictly required for the procedure, intraoperative OCT facilitates these maneuvers and the analysis of postsurgical outcomes (see Table 2). The intraoperative OCT improves the safety of subretinal injection by demonstrating depth in real time with high-contrast, high-resolution cross-sectional presentation rather than less precise anatomic clues of surface dimpling, retinal whitening, choroidal blanching, or bending of the injection cannula. This can allow for a gentler entry of the cannula and fluid into the subretinal space and reduce the risk for bleeding, inflammation, or choroidal neovascularization. The retina becomes opacified when it is detached, and after bleb initiation the tip of the subretinal cannula cannot be visualized directly until it is withdrawn from the retinotomy. With the intraoperative OCT, the retinotomy and cannula can be observed throughout the procedure to provide real-time feedback that may prevent inadvertent deeper penetration into the choroid, withdrawal into the vitreous cavity, or stretching of the retinotomy. For eyes treated under protocols requiring a pre-bleb, the initial retinotomy can sometimes be difficult to locate after exchanging injection syringes. Attempts at reentering the retinotomy may stretch it or create a second retinotomy, and this promotes reflux by creating a low pressure outlet into the vitreous cavity. The retinotomy is readily located with intraoperative OCT scanning even in the presence of limited preretinal bleeding from the injection site.
| All patients (n=26) | Intraoperative OCT-Aided (n=16) | No Intraoperative OCT (n=10) | |
| Median Age (Range) | 20.8 years (2.1 to 56) | 21.5 years (8.2 to 52.4) | 20.4 years (2.1 to 56) |
| Sex | 42.3% | 37.5% | 50% |
| Gene Affected | |||
| RPE65 | 11 (19 eyes) | 4 (6 eyes) | 7 (13 eyes) |
| RPGR | 4 | 3 | 1 |
| CNGA3 | 3 | 2 | 1 |
| CNGB3 | 8 | 7 | 1 |
Table 1. Baseline Demographics of Patients Who Received Subretinal Gene Augmentation Therapy
| Use of Intraoperative OCT for Gene Therapy | Complications Avoided |
| Document preinjection anatomy and foveal architecture | Failure to identify and document an anatomic change between baseline visit and the time of surgery |
| Confirm hyaloid elevation and absence of epiretinal membranes at injection site | Failure to initiate bleb, possibly wasting vector |
| Confirm correct layer is receiving subretinal therapeutic | Suprachoroidal or subhyaloid injection with ineffective treatment or wasted vector, Risks of subretinal or choroidal bleeding and inducing choroidal neovascularization |
| Monitor foveal status during bleb formation | Macular hole, failure of bleb to reach fovea |
| Confirm continued bleb advancement | Failure of bleb to propagate, reflux, macular hole |
| Document post-injection bleb location, size of retinotomy, and absence of leakage out of the retinotomy | Inadequate treatment due to reduced volume, inflammatory response from refluxed vector |
| Facilitate discussion among clinical trial sponsors, surgeons, and perioperative team in evaluating procedural and anatomic outcomes | Failure to demonstrate safety of procedure to regulatory agencies, failure of surgeons/consortiums to learn from surgical outcomes |
Table 2: Uses of Intraoperative OCT During Subretinal Gene Augmentation Surgery
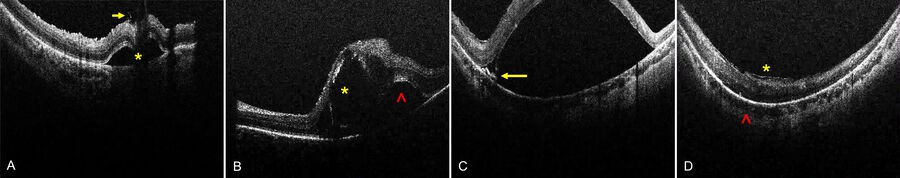
Subretinal injection of gene augmentation therapy is inherently traumatic, subjecting the photoreceptors and RPE to high pressures designed to open a potential space and inducing a localized rhegmatogenous retinal detachment (RRD). Excessive injection pressures have created permanent retinal and RPE damage in monkey models and human eyes [17-22]. This can be mitigated by footpedal controlled automatic injection systems, venting the vitreous cavity to reduce the pressure differential relative to the subretinal space, and continuous real-time monitoring of bleb expansion by intraoperative OCT. The fovea is the thinnest area of the retina and also the most critical for vision. Blebs involving the fovea place significant stress on foveal integrity, particularly when the retinotomy is initiated close to the fovea. Foveal integrity can be continuously monitored with intraoperative OCT, and the surgeon heeding the sign of foveal inversion can prevent foveal rupture into macular hole [23]. In cases of strong retina-RPE adhesion as in achromatopsia, the RPE layer may stretch profoundly resulting in a newly described surgical complication of residual RPE tension lines resembling incomplete RPE tear (Sisk RA, unpublished data). These tension lines can be observed clinically but are often obscured by the opacified detached retina of the bleb. However, they appear as progressive RPE elevations on intraoperative OCT that can signal the surgeon to reduce injection pressure or abandon further bleb expansion from that retinotomy.
Multiple reports demonstrate idiopathic RRD damages the photoreceptor layer with temporary or permanent loss of the ellipsoid zone and reduced visual acuity, and these findings are more severe with increased duration of RRD [20,24-26]. Subretinal fluid resolves within a few days after subretinal injection for gene therapy but visual recovery and anatomic recovery may take weeks or months. Some reports indicate failure to recover may result from higher injection
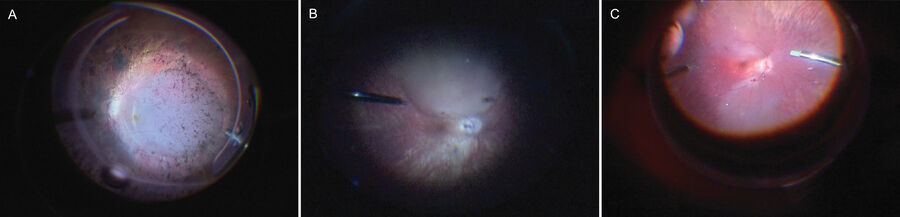
pressures or more advanced retinal degeneration at the time of surgery [10,18,22]. Patients with advanced LCA type 2 who received RPE65 gene therapy in the early clinical trials were reported to have postoperative retinal thinning and RPE degeneration [26-31]. Patients with choroideremia with foveal photoreceptors unsupported by underlying RPE sometimes developed foveal atrophy with worsening visual acuity [32,33]. Bullous foveal detachment delays resorption of subretinal fluid beneath the fovea, prolongs foveal ischemia by separation from the choroidal circulation, and may expose the fovea and parafovea to trauma from excessive injection pressures. In situations where the macula needs treatment but the fovea is vulnerable, intraoperative OCT can monitor advancement of the bleb to just reach the fovea or facilitate kissing blebs that just spare the foveal center (Figure 2).
Practical Considerations of Intraoperative OCT in Gene Augmentation Therapy
The addition of intraoperative OCT to the workflow of the surgery room requires a collaborative approach from the surgical team. Although the technology is designed to be surgeon-driven, it may be challenging or distracting for the surgeon during certain maneuvers to primarily control the intraoperative OCT. In those situations, a trained assistant with knowledge of the steps and goals of the surgical procedure, who is proficient with the intraoperative OCT controls and in close communication with the surgeon, can direct intraoperative OCT visualization to the surgeon seamlessly without distracting from a critical surgical maneuver. The surgeon can resume operation of the intraoperative OCT at any point via foot-pedal control if the target tissue is not be adequately viewed. For gene augmentation therapy, bleb initiation is the most delicate maneuver in the procedure, and it can be helpful to utilize an assistant to acknowledge formation of the bleb and the layer into which fluid is injected. Once the bleb is initiated, the surgeon can resume control and make adjustments to the microscope or intraoperative OCT plane as the bleb rises.
There are multiple options for intraoperative OCT scanning, including live single or crosshair rasters or volume scanning patterns (circular, rectangular, or annular) that may be rotated in any direction and adjusted for size and scan resolution. Both anterior and posterior segment imaging are possible, although adjustments are needed when changing between widefield and contact viewing systems for posterior segment surgery. For pediatric patients or adults who are unable to cooperate with examination in the clinic prior to surgery, scanning can even be performed before surgery even without microscope illumination, although illumination may improve the quality of the reference image for the OCT. Otherwise, such maneuvers can be performed at the beginning of the surgical procedure. During gene augmentation therapy procedures, in order to be timeefficient, posterior segment baseline imaging can be performed during the core vitrectomy with instruments kept in the safe zone centrally but not obscuring the posterior segment pathology of interest. At the end of the procedure, similar scans can be obtained as the vitreous cavity is washed of refluxed vector by fluid-fluid exchange. During subretinal injection, the preferred intraoperative OCT scanning technique places the horizontal raster at the retinotomy site and the vertical raster at the fovea to monitor stress on the fovea as the bleb enlarges.
Real-time OCT images can also be viewed in a variety of ways: image injection, external monitor, or integrated with heads-up 3D surgery. For surgeons without access to 3D visualization systems, image injection into the microscope ocular provides real-time raster images positioned away from the central field of view. The image quality is reduced compared to projection onto external monitors or 3D systems, but is sufficient for membrane peeling and subretinal injection procedures. The raster scans may also appear bright in the background when overlapping posterior segment structures. External monitors and 3D visualization systems engage the surgical team to follow the procedure and anticipate surgeon needs (Figure 3). The intraoperative OCT scans are presented at full resolution, although the information is variably displaced from the view of the surgeon’s instruments. With external monitors, the degree of displacement depends upon the position of the monitor relative to the surgeon’s view through the oculars. With 3D visualization systems, the image can be presented either on an adjacent monitor without sacrificing resolution of the surgical field, a side-by-side image on a single monitor, or as a picture-in-picture that may be manipulated for size and position that obscures a selected portion of the surgical field.
Surgical video and OCT data are routinely collected in gene augmentation therapy procedures to validate delivery of the vector to the target treatment area for the sponsors of clinical trials and to correlate anatomic and functional consequences of the surgery or the vector for safety and efficacy analysis. Recordings can include microscope video only, the live intraoperative OCT feed only, simultaneous recordings of intraoperative OCT and the registration surgical video, microscope video with picture-in-picture intraoperative OCT overlay, or any combination of these options. Surgical and OCT video can facilitate discussion among gene therapy surgeons, sponsors, and advisory and regulatory boards. In the era of COVID-19, video-based proctoring may suffice as training for new gene therapy surgeons.
Endorsement
The Leica Proveo 8 is a superior platform for subretinal gene augmentation treatment delivery during surgery. The scan depth is sufficient for either widefield viewing system or contact lens-based applications, and at the time of this publication, the maximum intraoperative OCT scan resolution exceeds any commercially available competitor. This provides excellent visualization of subsurface details throughout the procedure. The real-time intraoperative OCT has customizable controls integrated into the microscope foot pedal that readily allow facile adaptations for the surgical procedure or deference to an assistant intraoperative OCT operator depending upon surgeon preference. The Proveo 8 uniquely allows independent control of the microscope and OCT focus and position compared to other commercially available intraoperative OCT capable microscopes. As the bleb expands and the depth of the retinotomy relative to the fovea may differ, this is especially useful for monitoring foveal stress independent of the retinotomy. The surgical video produced by the Proveo 8 is presentation/publication quality, and the platform offers all of the aforementioned choices for recording and sharing surgical and OCT video as well as volume scan data.
Intraretinal blebs tend to track into the subretinal space as they expand. C) RPE tension lines (yellow arrow) at the base of a bullous bleb indicate strong retinal-RPE adhesion and risk for further stretching with permanent RPE changes. D) Epiretinal membranes (yellow asterisk) may limit bleb creation. Increased posterior curvature (red caret) may promote high bleb formation and risk for foveal inversion and macular hole. Images courtesy of Robert A. Sisk, MD, FACS, Cincinnati Eye Institute.
Disclaimer: This document contains viewpoints and experiences expressed by Robert A. Sisk MD, FACS and do not necessarily reflect the opinion of any institution with whom they are affiliated.
References
- Int J Mol Sci. 2019 Nov; 20(22): 5722. PMID: 31739639
- Gene Ther. 2012 Feb;19(2):154-61. doi: 10.1038/gt.2011.161. Epub 2011 Oct 27. PMID: 22033465
- Cold Spring Harb Perspect Med. 2015 Mar; 5(3): a017293. PMID: 25359548
- Pharm Res. 2019 ; 36(2): 34. doi:10.1007/s11095-018-2564-5. PMID: 30617669
- Hum Gene Ther. 2016 Aug 1; 27(8): 563–579. PMID: 27178388
- Eye (2017) 31, 1308–1316. PMCID: PMC5601444
- J Clin Invest. 2019 Nov 1; 129(11): 4901–4911. PMID: 31408444
- Mol Ther Methods Clin Dev. 2020 Mar 13; 16: 179–191. PMID: 32055646
- PLoS One. 2011; 6(2): e17140. PMID: 21347253
- Ophthalmology 2019;126:1273-1285 PMID: 31443789
- Lancet. 2017 Aug 26;390(10097):849-860. PMID: 28712537
- Lancet. 2016 Aug 13;388(10045):661-72. PMID: 27375040
- N Engl J Med 2015;372:1887-97. PMID: 25938638
- Ophthalmology 2016;123:1606-1620. PMID: 27102010
- Eye (2017) 31, 1308–1316. PMID: 28820183
- Ophthalmol Retina. 2020 Jun;4(6):644-645. PMID: 32387052
- Ophthalmic Res. 2017;58(4):217-226. PMID: 28858866
- Graefe’s Arch Clin Exp Ophthalmol (2002) 240:232–237. PMID: 11935282
- Arch Ophthalmol. 2012 January ; 130(1): 65–75. PMID: 21911651
- Ophthalmol Vis Sci. 2014;55:6575–6579. PMID: 25190655
- Invest Ophthalmol Vis Sci. 2017;58:4155–4160. PMID: 28829847
- PLoS One. 2018 Dec 31;13(12):e0209996. PMID: 30596769
- N Engl J Med 2008;358:2240-8. PMID: 18441370
- Invest Ophthalmol Vis Sci. 2016;57:889–898. PMID: 26943151
- Invest Ophthalmol Vis Sci. 2015 Jan 22;56(2):1040-50. PMID: 25613940
- PLoS One. 2017 Sep 13;12(9):e0184783. PMID: 28902881
- Lancet 2009; 374: 1597–605. PMID: 19854499
- N Engl J Med 2015;372:1887-97. PMID: 25938638
- N Engl J Med 2015;372:1920-6. PMID: 25936984
- Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):E517-25. PMID: 23341635
- Arch Ophthalmol. 2012 Jan;130(1):9-24. PMID: 21911650
- Expert Rev Ophthalmol. 2018;13(3):129–138. PMID: 31105764
- Lancet 2014; 383: 1129–37. PMID: 24439297