This procedure requires high levels of precision. As such, intraoperative Optical Coherence Tomography (OCT) is an essential tool. It provides ophthalmic surgeons with real-time information during surgery, supporting bleb management and allowing confirmation that the desired volume of vector reached the subretinal space.
Intraoperative OCT can also help prevent complications such as the formation of an intraoperative macular hole during vector injection.
Case description
A 21-year-old Hispanic female had poor vision from birth including nyctalopia, visual field constriction, reduced visual acuity and reduced color perception. She noticed slowly progressive functional decline.
Although she completed high school, she had worsening navigation and was challenged to live and work independently due to her visual limitations.
Diagnosis & pre-operative assessment
Pre-operative visual acuity was 20/150 in both eyes. The patient exhibited typical diffuse retinal degeneration with relative macular sparing. Genetic testing revealed biallelic RPE65 variants causing retinal degeneration and indicating type 2 Leber congenital amaurosis. Based upon the presence of viable retinal cells and potential benefit from gene therapy, treatment was offered by subretinal injection for both eyes. Surgeries were performed two weeks apart.
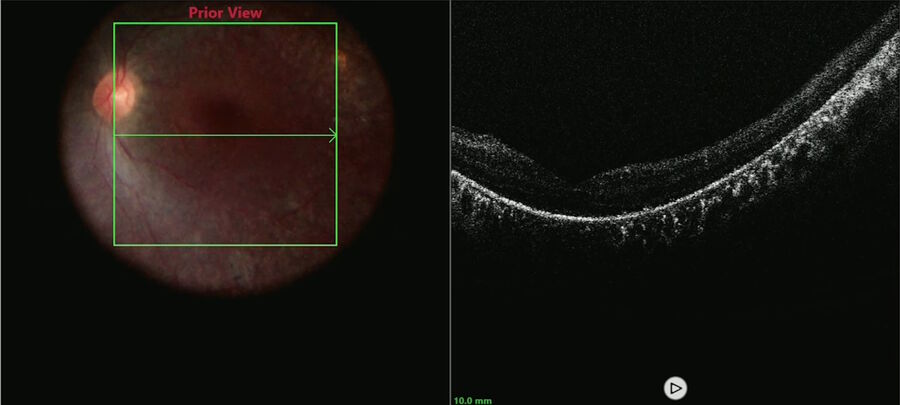
The macular OCT and ultra-wide field fundus images showed baseline pigmentary retinopathy and photoreceptor alterations relatively sparing the fovea (Figure 1).
Surgical approach
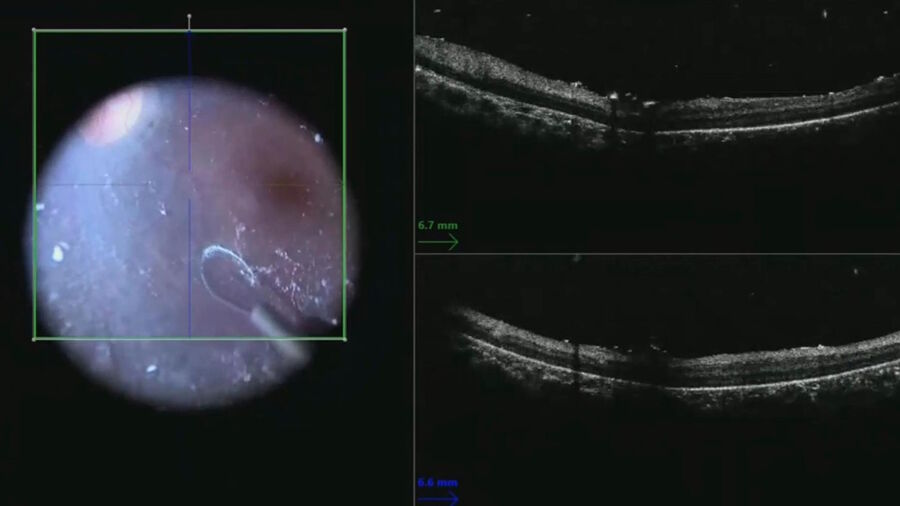
A macular scan was performed to document the pre-injection anatomy and look for vitreous or epiretinal membranes that could interfere with bleb formation or create post-operative tractional complications (Figure 2).
After completing the core vitrectomy, dilute triamcinolone was injected to stain the hyaloid. The hyaloid face is often split in these cases and may require manual elevation rather than aspiration off the optic disc.
The live intraoperative OCT image was helpful for illustrating the plane between the retina and the hyaloid face (Figure 3). The nitinol loop was used like a pick to elevate the hyaloid face from the macula rather than initiating the hyaloid elevation at the disc. After cortical vitreous removal, a scleral depressed examination was done to search for retinal breaks.
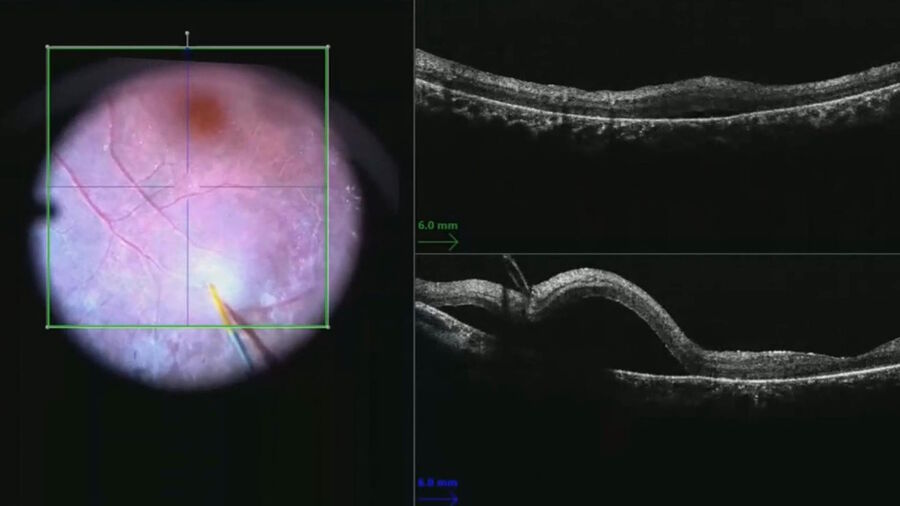
Then, to cover the macula, a beveled extendible subretinal cannula was used inside the superotemporal arcade. The injection was done slowly while venting one cannula to prevent build-up of pressure within the vitreous cavity and subretinal space that could have inhibited delivery of vector.
The bleb was created with the lowest pressure possible and slowly expanded while decreasing the injection pressure. The injection flow was then reduced when approaching the fovea to allow pressures to equilibrate and avoid placing excessive strain on the fovea that could risk macular hole formation. The foveal anatomy was followed in real time with the intraoperative OCT (Figure 4).
As the bleb enlarged, the surgeon could follow the inverted image and adjust the z-axis to better monitor the fovea. An assistant was useful in this portion of the procedure to make adjustments on the intraoperative OCT to help the surgeon maintain a stable cannula position within the retinotomy.
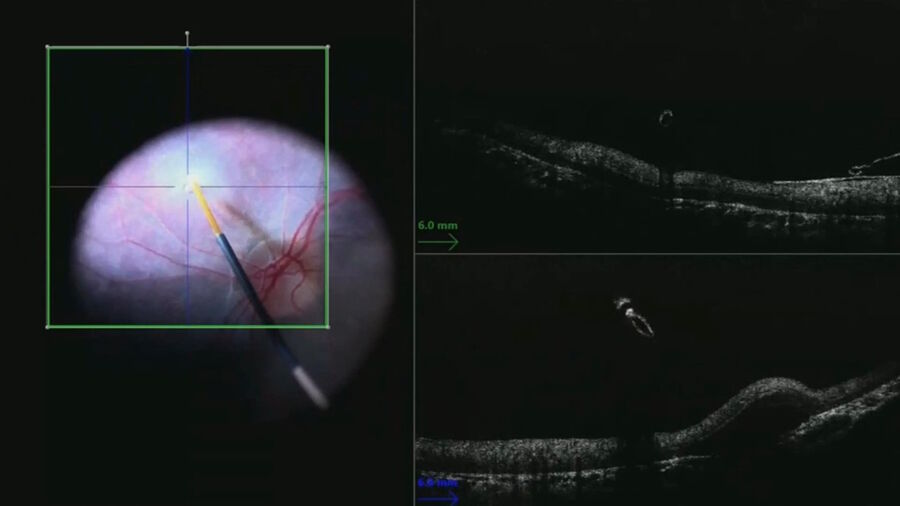
In this case, the bleb covered the entire macula. In order to cover the greatest area and provide more temporal visual field, the treatment was also applied nasal to the optic nerve (Figure 5).
Following the injections, the bleb dimensions were documented by tracing the margins on the surgical video and measurements with intraoperative OCT.
Post-operative outcomes
One month after the operation, visual acuity was unchanged, which is typical for adults receiving this type of gene therapy. The macular architecture was preserved, with no inflammatory complications. Visual sensitivity improved greatly. The patient is now able to navigate independently in dim light and reported seeing star light for the first time. She also has improved color vision.
Follow-up usually occurs 4 to 6 months after surgery to compare outcomes to baseline in patients receiving gene therapy treatments. This includes repeating visual field and full-field sensitivity testing.
Conclusions
The live intraoperative OCT image was critical to meet surgical goals and enhance patient safety as the subretinal injections for gene therapy were performed.
Intraoperative OCT provides the most accurate map of where the vector was distributed initially. This is essential for correlating changes in anatomy and visual sensitivity over time, whether positive or negative, to determine benefit from the treatment or harm from the surgical technique. The closer the retinotomy is to the fovea, the more likely and rapidly it will propagate posteriorly to the fovea.
This case demonstrates the utility of intraoperative OCT in confirming entry into the subretinal space rather than the preretinal or suprachoroidal spaces. It shows how to monitor bleb expansion and foveal integrity. It also demonstrates the value of post-injection scans to document the extent of the blebs, which can migrate after fluid-air exchange.