Case Description
A 42-year-old Caucasian male patient was diagnosed with a biallelic mutation in the RPE65 gene, causing Leber congenital amaurosis. He had advanced retinal dystrophy. He was working in administration, had a guide dog and found it difficult to walk at twilight.
Pre-operative assessment
The patient’s visual acuity pre-operatively was very poor and he had myopia. A full screening was performed showing an absent electroretinogram (ERG) and including a full-sensitivity test (FST) and multi-luminance mobility test (MLMT).
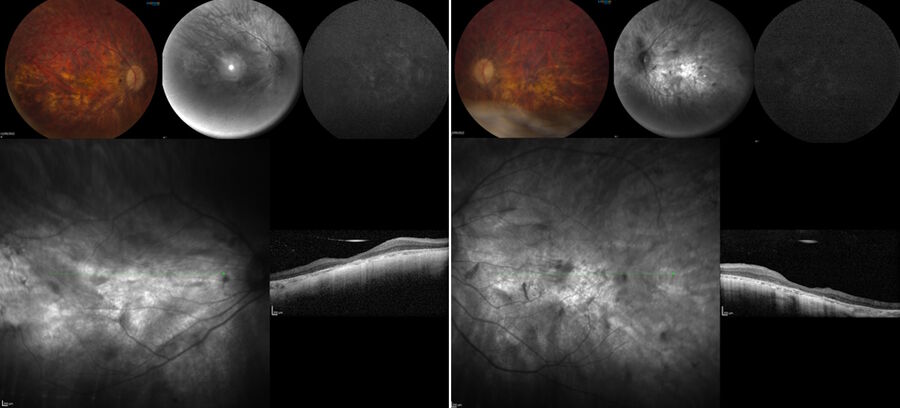
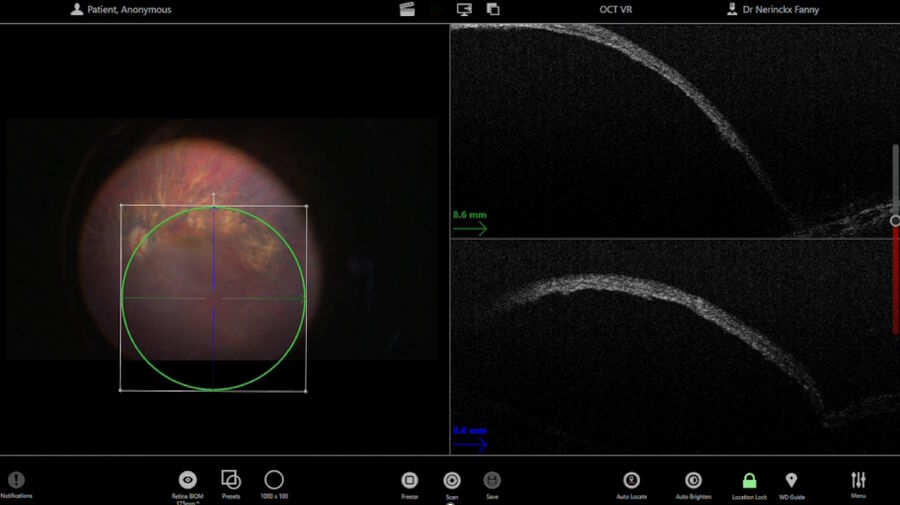
The pre-operative OCT and blue auto-fluorescence fundus images showed photoreceptors only in the foveal region and a symmetric left eye.
Surgical approach
The surgical plan was as follows: perform a 23g vitrectomy, stain the vitreous with diluted triamcinolone, conduct prophylactic peripheral laser treatment to avoid secondary retinal detachment and inject subretinally 300 microliters of gene therapy, using a microinjection system.
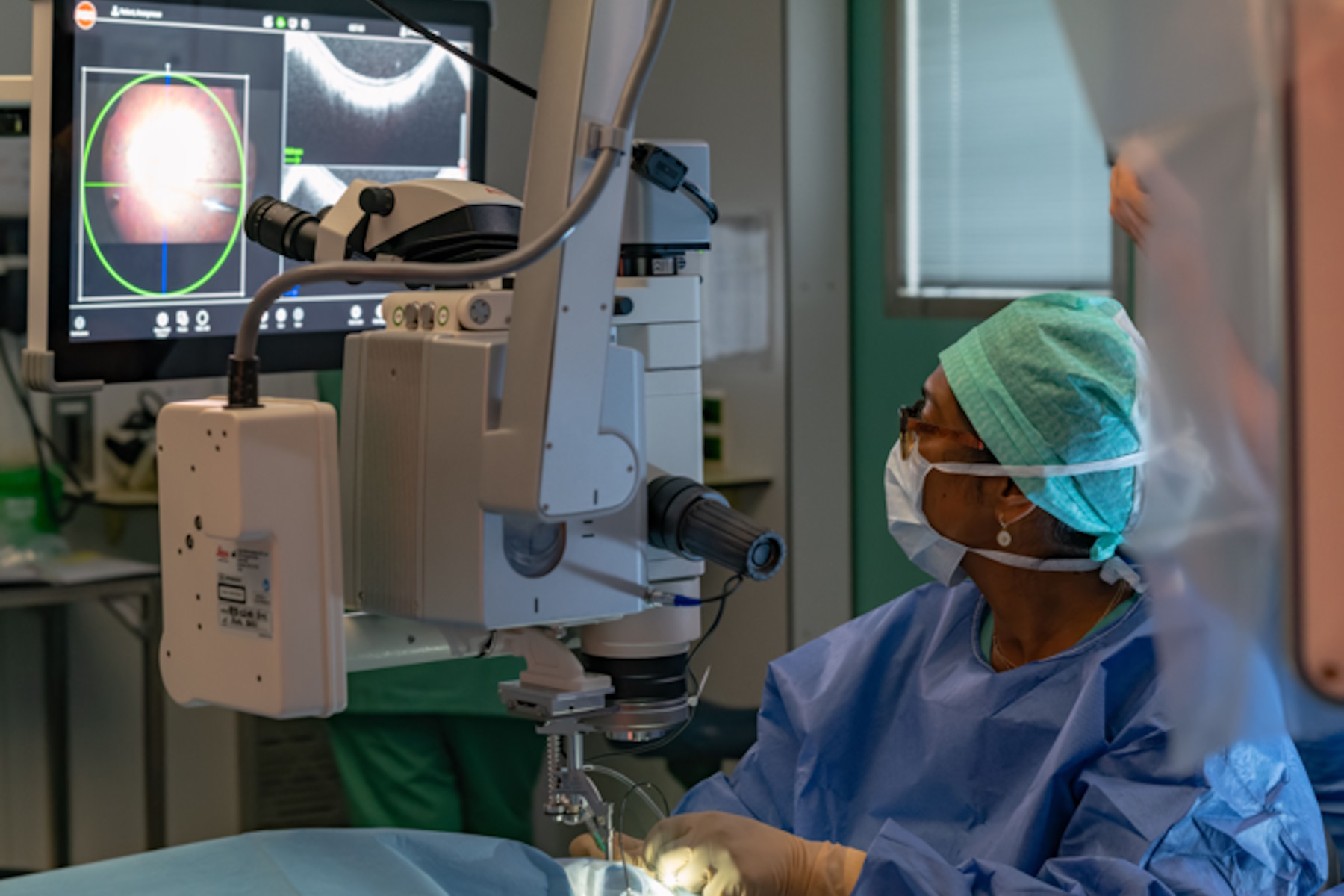
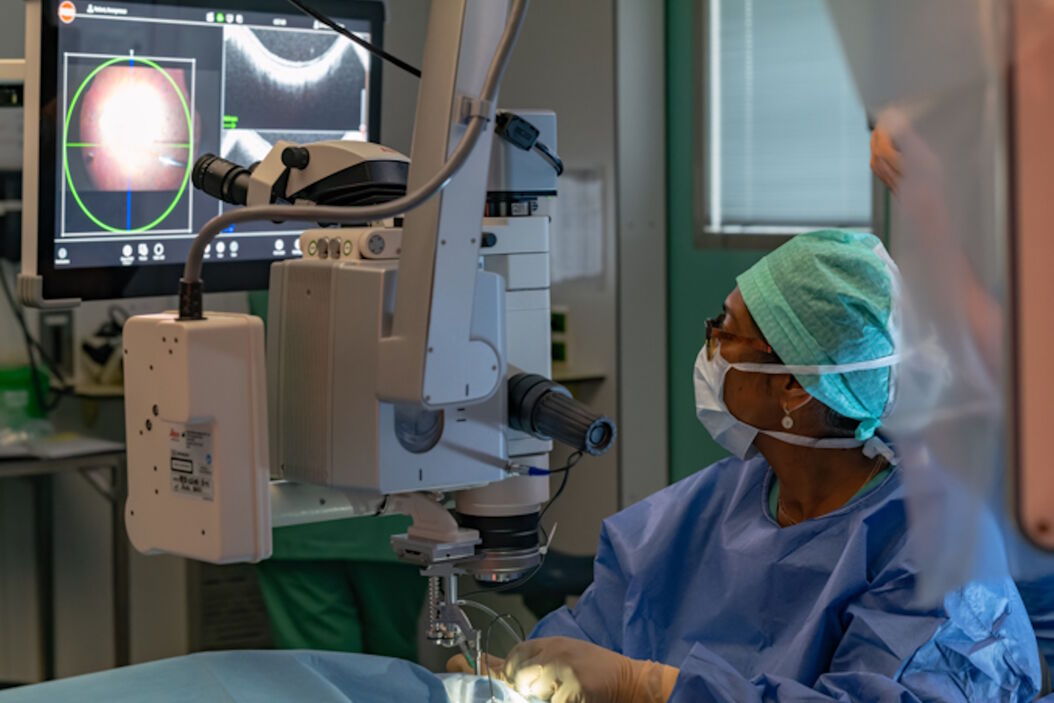
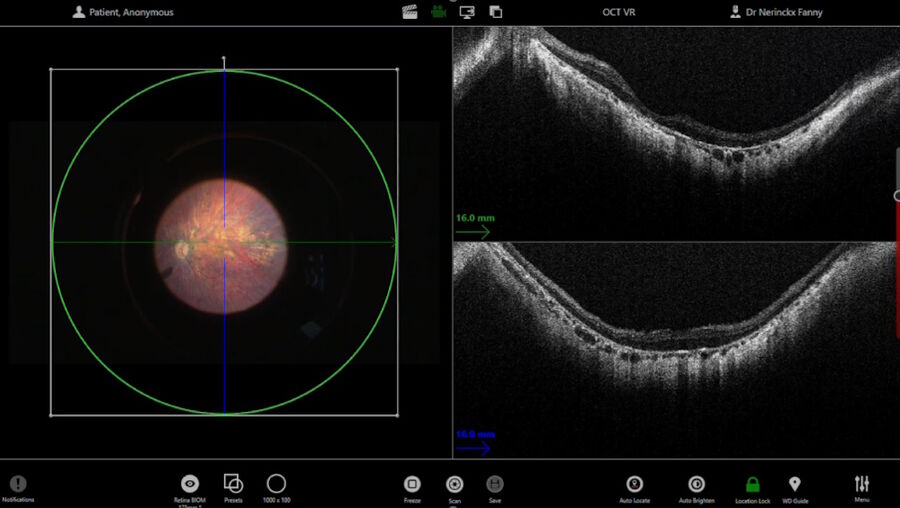
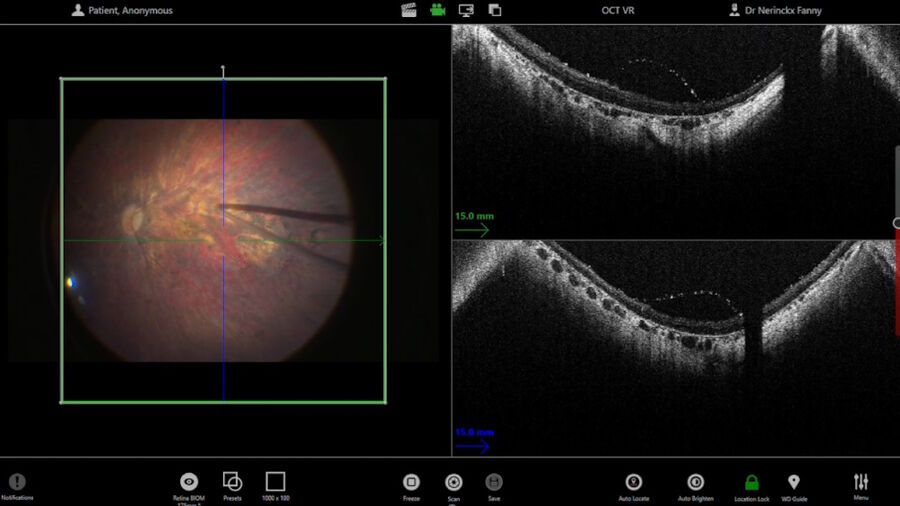
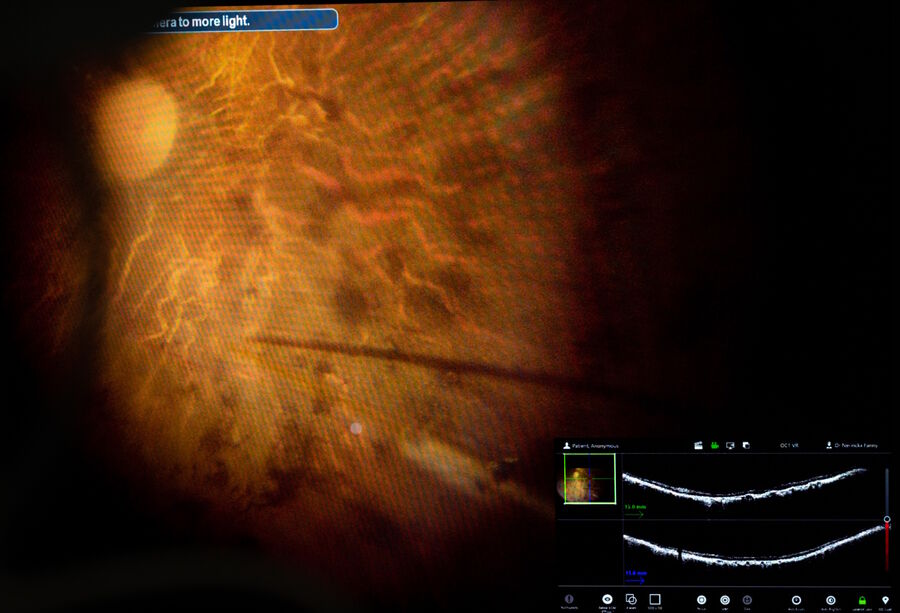
The intraoperative OCT allowed to check for posterior vitreous detachment in the right eye. It is controlled with the foot pedal and has an auto-locate function (Figure 2).
To check if any posterior hyaloid was left, triamcinolone was injected. A new scan was then performed in the macular region. The auto-locate showed some particles of triamcinolone, that were detached with an ILM forceps. The scan also showed vitreoschisis, which is common in adult patients. Without intraoperative OCT, this could have been missed.
Pulling on the remnants of the posterior hyaloid, it was lifted with a vitrectomy probe to detach it up to the periphery of the vitreous base. Shaving of the vitreous base was performed during the peripheral laser treatment.
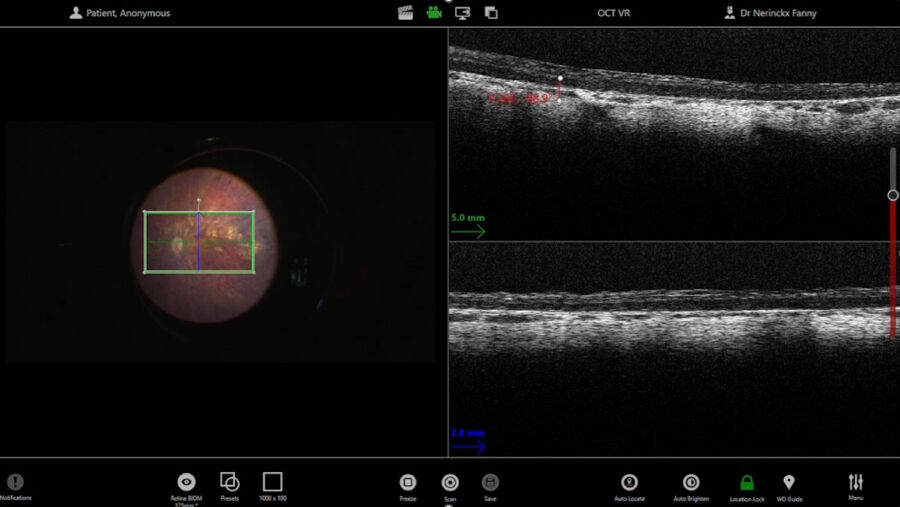
Before the subretinal injection, the surgeon measured the retinal thickness and inserted the subretinal cannula through the trocar. The intraoperative OCT helped to check the position of the cannula. The injection was performed with a microinjection system. Once the bleb was induced, the pressure was reduced to make the bleb grow very slowly and softly up to the fovea. The use of intraoperative OCT supported control of the bleb throughout.
The 300 microliters of gene therapy were injected under the retina. The intraoperative OCT foot pedal allowed to move the scan as needed.
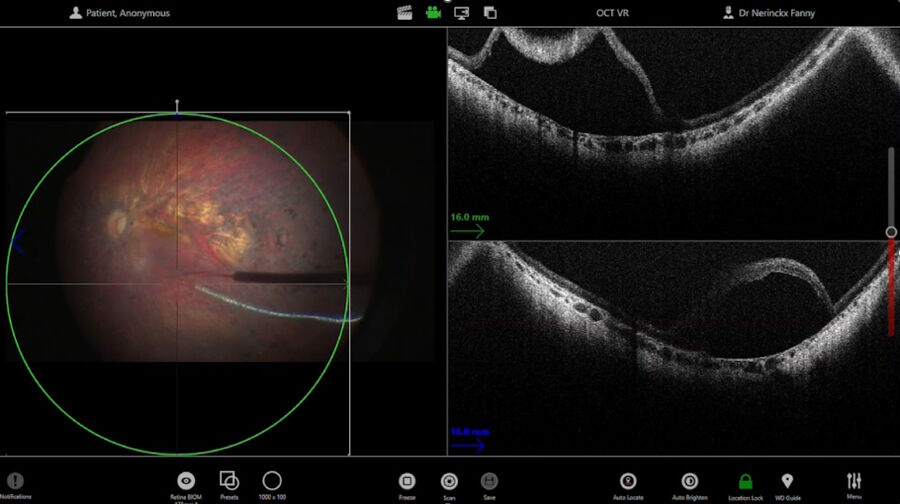
Once the bleb was fully created and all the fluid injected, several scans were performed and recorded for clinical trial purposes. The scan of the surface of the bleb allowed to check for foveal stretching and confirm there was no macular hole. The injection side was also verified, to ensure the retinectomy was closed.
At the end of the procedure, a BSS fluid exchange was performed, allowing to remove any therapy that would have escaped into the vitreous, clean the vitreous and avoid inflammatory reactions. This mechanical exchange may also contribute to move the bleb posteriorly a little bit, to ensure all the posterior segment has been treated.
Post-operative outcome
After surgery, as the patient had advanced dystrophy, the visual acuity only improved slightly. However, the patient reported better vision at twilight. He also indicated that he was now able to look at people’s faces at dinner and distinguish shapes of cars in the street in the evening. In addition, he shared that he no longer stared at lights.
Conclusions
The use of intraoperative OCT during RPE65 gene therapy surgery has numerous benefits. It allows to ensure removal of epiretinal tissue, measure retinal thickness and supports real-time positioning of the subretinal cannula tip. It also essential to monitor subretinal bleb and fovea stretching and check retinotomy closure.
The picture in picture integration of OCT into the 3D heads up screen supports concentration and focus during surgery, especially when holding the cannula under the retina.
With intraoperative OCT, the full team in the operating room can follow the case and exchange as needed. The technology integrates seamlessly in the surgical flow.
Disclaimer: Please note that off-label uses of products may be discussed. Consult with regulatory affairs for cleared indications for use in your region. The statements of the healthcare professionals included in this presentation reflect only their opinion and personal experience. They do not necessarily reflect the opinion of any institution with whom they are affiliated or Leica Microsystems.