The challenge of visualizing living samples
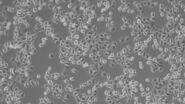
Brightfield microscopy often only provides a weak image of unstained specimens, in which only a few details are detectable. A stronger contrast is important to see more details in the picture. One way to enhance contrast is to stain the sample. As this is not possible for living organisms, however, they stay uncolored and appear inconspicuous. These specimens actually interact with the incident light in an optical microscope as well. Yet a phase shift occurs that is not detectable with the human eye. The eye can only experience changes in amplitude (brightness) and in frequency (color).
Contrast refers to the possibility to distinguish between the sample and the background and to discover details in the sample. It is defined as the difference in light intensity between the image and the adjacent background compared to the overall background intensities. For the human eye these differences need to be at least two percent to be detectable. Great improvements may be achieved using photo detectors.
However, the contrast is not only determined by the specimen and its interaction with light itself. The optical system which is used to observe the specimen and its ability to record the image information is critical as well. In a microscopic system, contrast depends on the right aperture settings, the grade of the optical aberration, the contrast method used, the specimen and the detector.
The contrast of an image acquired with an optical microscope can therefore be influenced independently of contrast methods by changing the aperture settings of diaphragms. But if for example the condenser is stopped down too far, the resolution will be impaired and diffraction artifacts may appear.
The oldest method of achieving adequate contrast is probably to stain the sample first. Commonly, this is only possible for dead material and occasionally requires some complicated staining protocols. If one wants to observe living cells, staining is not easily done. Therefore different techniques – provided by an adequate optical microscope – aim to change the phase shift, which is caused by the interaction of light with the specimen, into an amplitude shift.
Phase contrast and differential interference contrast (DIC) are examples of these contrast methods. Other optical contrast methods are darkfield and polarization contrast.
Interaction between light and specimen
If incident light strikes a specimen, they will interact. Absorption, reflection, diffraction, light scattering and refraction are possible consequences. During this process the striking light waves are changed. Phase shifts as well as changes in amplitude are possible.
In this respect one can distinguish between phase and amplitude objects. Depicted ideally, phase objects are samples that change the phase but not the amplitude of a light wave. In contrast, amplitude objects only affect the amplitude but not the phase of light. Flat and unstained cells almost reach the characteristics of a phase object for visible light. As they just lead to a phase shift which cannot be seen by the human eye, their contrast in brightfield microscopy is very low. Amplitude objects themselves can be imaged very well in brightfield microscopy. They reduce the amplitude and therefore the intensity of light passing through.
Stained or naturally colored objects can be displayed very well in a bright field. They belong to the amplitude objects. They diminish the light passing through and consequently reduce the amplitude of the wavefronts. But they are not ideal amplitude objects. Besides the amplitude, they also influence the composition of the striking light. They absorb or reflect waves with a distinct frequency, whereas light of other wavelengths can pass through unhindered.
Phase shifts occur because of the different refraction indices of the specimen and the surrounding medium. If a light wave enters the cell it will be quasi decelerated while the amplitude will stay the same. As light leaves the cell it recovers speed and extends through the medium with the same frequency, wavelength and amplitude as before. Yet its phase has shifted compared to light which has only crossed the medium.
Given that the human eye and other optical detectors are not able to recognize and detect phase shifts of light waves, the main aim of the contrast methods in the case of unstained and uncolored specimens is to generate an amplitude contrast and to change a phase contrast into an amplitude contrast.
Great impact on biological research
The use of unstained specimens offers many advantages compared to the use of fixed and stained objects. It allows the observation of living objects in a biological microscope, which are devoid of fixation and staining artifacts. Fixation and staining can for example make the specimen shrink or swell. Furthermore, there is the risk of destroying distinct structures in the cell or substantially changing their morphology. The only sure way of eliminating this danger is to use living samples, which are free of artifacts and provide reliable and authentic information.
Another great advantage of live cell microscopy is the examination of time-dependent scenarios. Besides gaining static information of the cell and a snapshot of its situation, whole processes can be readily monitored, e.g. cell motility, cell division and also apoptosis. As these processes may occasionally take a lot of time, it can be advisable to add a camera to the optical microscope system for the recording of time lapse movies.
The information and impressions obtained from the different optical contrast methods vary depending on their origin. It is therefore advisable to rely on a combination of different contrast techniques to get the most accurate and detailed image of the examined specimen. Whereas brightfield often does not allow exact observation of the cell’s morphology, other optical contrast methods may provide further insights.
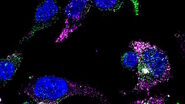
More information can be obtained by adding fluorescent markers to the live image of the cells. A protein of interest in the cell can be tagged with a fluorescent protein like GFP. Its localization can then be traced and exactly determined inside the cell via the light microscopic images. There is also the possibility to couple GFP to a peptide location signal. In this way distinct compartments like the endoplasmic reticulum can be visualized.
Modern optical microscopes used in research usually have a modular design, which enables fast switching between the individual contrast methods or even their simultaneous application. Optical contrast methods give the potential to easily examine living and colorless specimens in everyday laboratory routine.